One mole of an ideal gas is compressed from 500cm^3 against a constant pressure of 1.216 × 10^5 Pa. The work involved in the process is 36.50 J. Calculate the final volume.

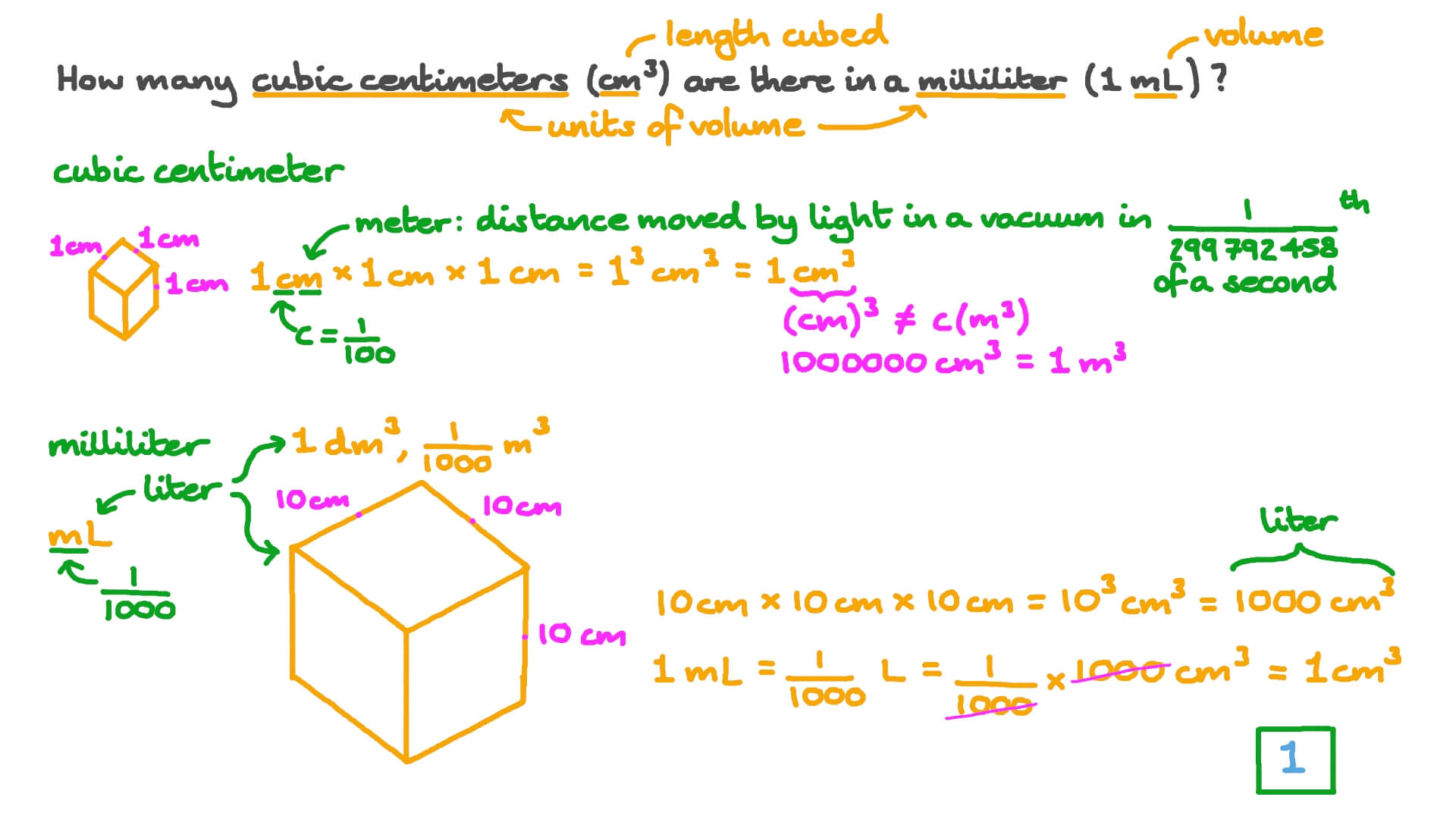
Mole Calculations. Volume, cm 3 Mass, gMolesAtoms use densityuse molar mass use Avogadro's number g cm 3 mol g atoms mol xxx A graduated cylinder holds. - ppt download
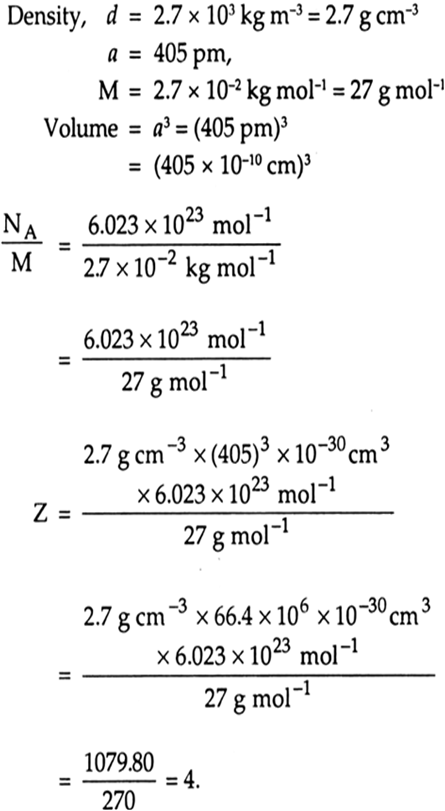
A cubic unit cell with an edge length of a cm consists of identical particles. The mass of each particle is m g and the density of the unit cell is (4m)/a^(3)g*cm^(-3).

Mole Calculations. Volume, cm 3 Mass, gMolesAtoms use densityuse molar mass use Avogadro's number g cm 3 mol g atoms mol xxx A graduated cylinder holds. - ppt download

Free Energies, a Equilibrium Constants, and Number of Complexes Per... | Download Scientific Diagram
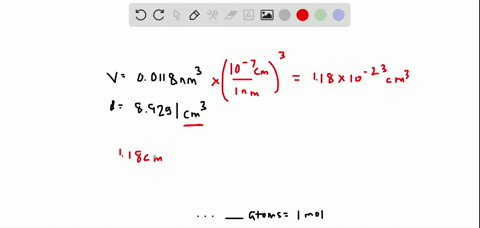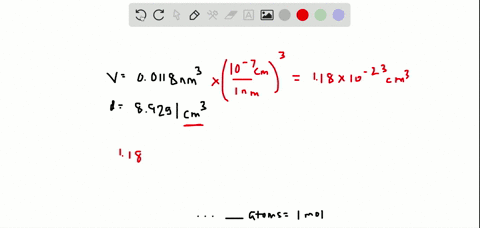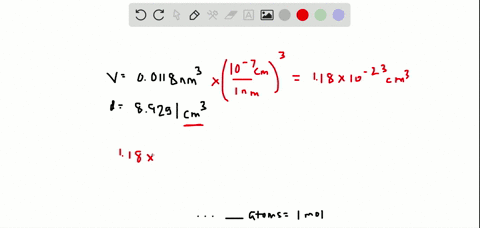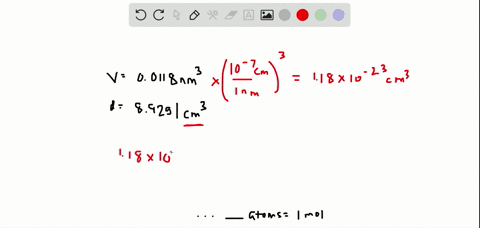
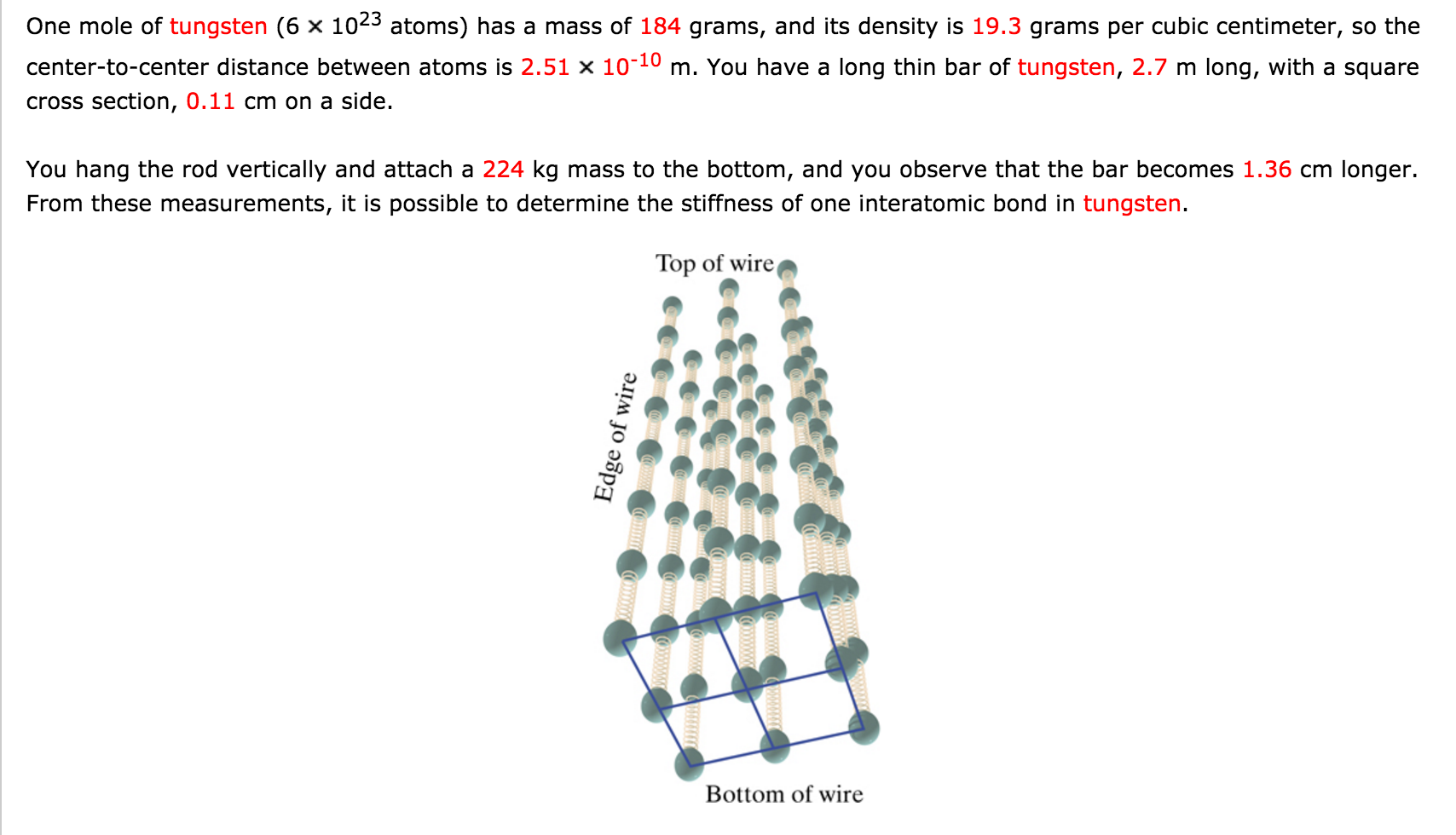
SOLVED:The volume occupied by each copper atom in a 1 -mole crystal is 0.0118 nm^3. If the density of the copper crystal is 8.92 g / cm^3, what is the experimental value

Sensitivity (in cm 3 ·mol −1 per 0.01 log unit) of the calculated V 1 °... | Download Scientific Diagram
The molarity of H2SO4 is 0.8 and its density is 1.06 cm^3. What will be its concentration in terms of molarity and mole fraction? - Quora