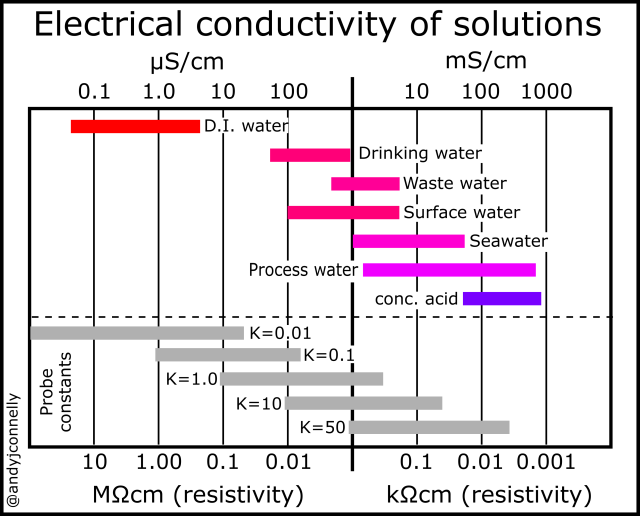
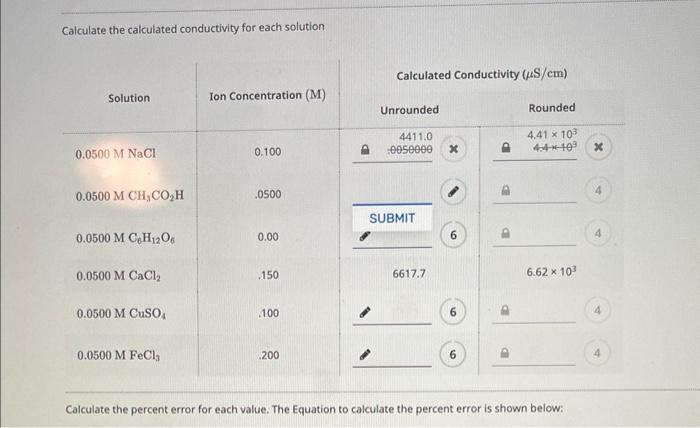
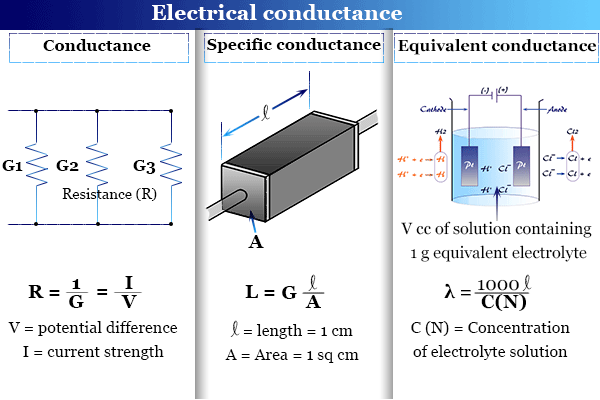
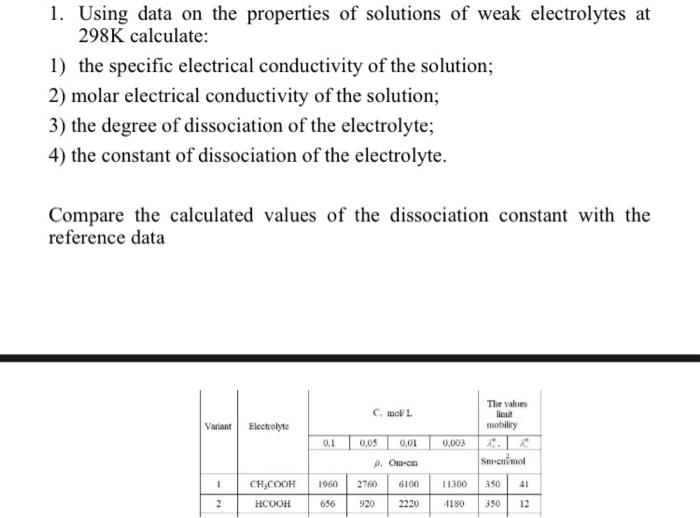
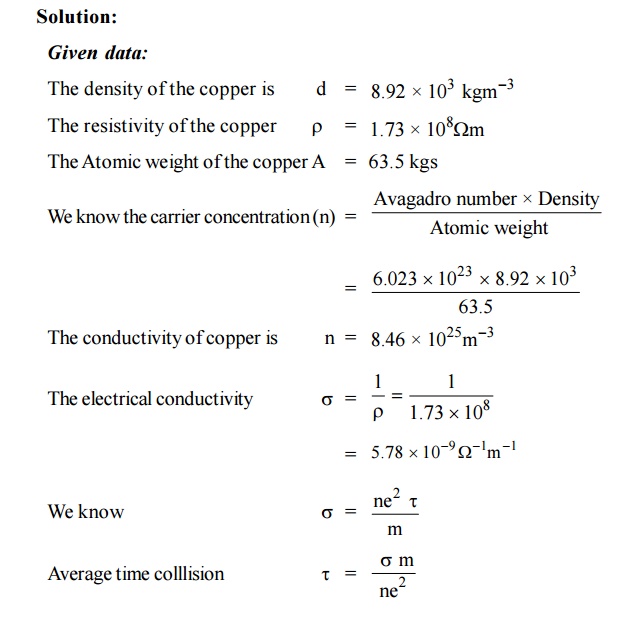
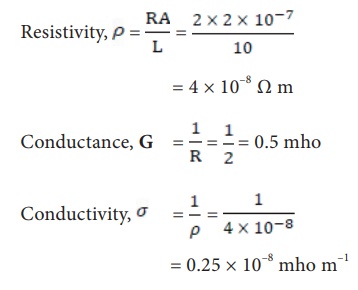a) Define the terms conductivity and molar conductivity for the solution of an electrolyte. Comment on their variation with temperature (b) The measured resistance of a conductance cell was 100 ohms. Calculate (
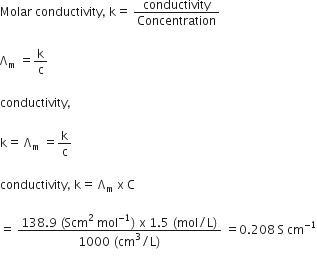
The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9S cm2mol-1. Calculate the conductivity of the conductivity of this solution. from Chemistry Electrochemistry Class 12 UP Board

the conductivity of a solution containing 1 04g of anhydrous BaCl2 in 250ml of water has been found to be - Chemistry - Electrochemistry - 13781839 | Meritnation.com
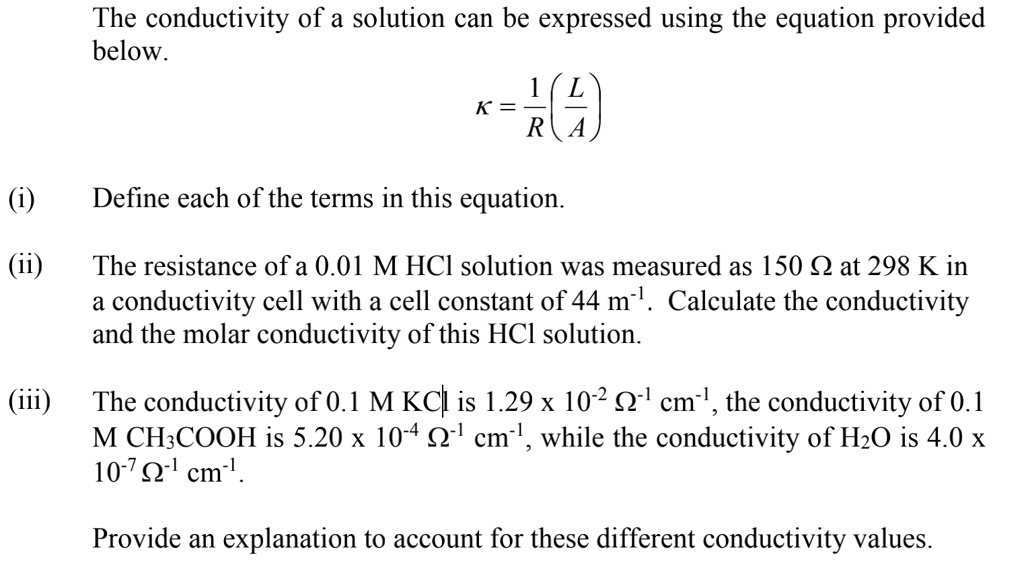
SOLVED: The conductivity of a solution can be expressed using the equation provided below. K = R(4) R Define each of the terms in this equation. (ii) The resistance of a 0.01

The molar conductivity of a 1.5 M solution of an electrolyte is found to be `138.9 S cm^(2) mol^(-1) - YouTube

The specific conductivity of a solution containing `1.0g` of anhydrous `BaCI_(2)` in `200 cm^(3)` of - YouTube
![SOLVED: Calculate the transierence number, and conductivity of a solution of 0.05 M KOH al room tempereture with the following data given. 00smKoh Imal m ] 90485 Icculomtmall 8 314 Mmol KI 298 15 0 007352 Im*0 mall 0,04978 Im*0 moll SOLVED: Calculate the transierence number, and conductivity of a solution of 0.05 M KOH al room tempereture with the following data given. 00smKoh Imal m ] 90485 Icculomtmall 8 314 Mmol KI 298 15 0 007352 Im*0 mall 0,04978 Im*0 moll](https://cdn.numerade.com/ask_images/231ab7c3cec045fa87af90022f3b9bf2.jpg)
SOLVED: Calculate the transierence number, and conductivity of a solution of 0.05 M KOH al room tempereture with the following data given. 00smKoh Imal m ] 90485 Icculomtmall 8 314 Mmol KI 298 15 0 007352 Im*0 mall 0,04978 Im*0 moll