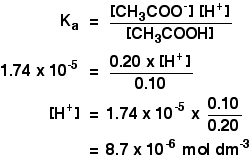![Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ] Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/dDlCNVZnUE9URzQ=/sd/)
Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]
![Calculate the pH of buffer solution containing 0.05mol NaF per litre and 0.015 mol HF per litre. [Ka = 7.2 xx 10^(-4) for HF]. Calculate the pH of buffer solution containing 0.05mol NaF per litre and 0.015 mol HF per litre. [Ka = 7.2 xx 10^(-4) for HF].](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/643088122_web.png)
Calculate the pH of buffer solution containing 0.05mol NaF per litre and 0.015 mol HF per litre. [Ka = 7.2 xx 10^(-4) for HF].
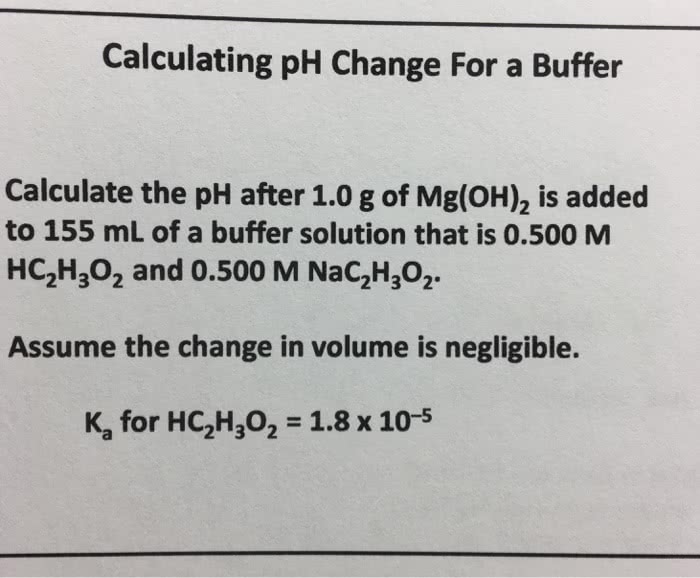
OneClass: Calculating pH Change For a Buffer Calculate the pH after 1.0 g of Mg(OH)_2 is added to 155...

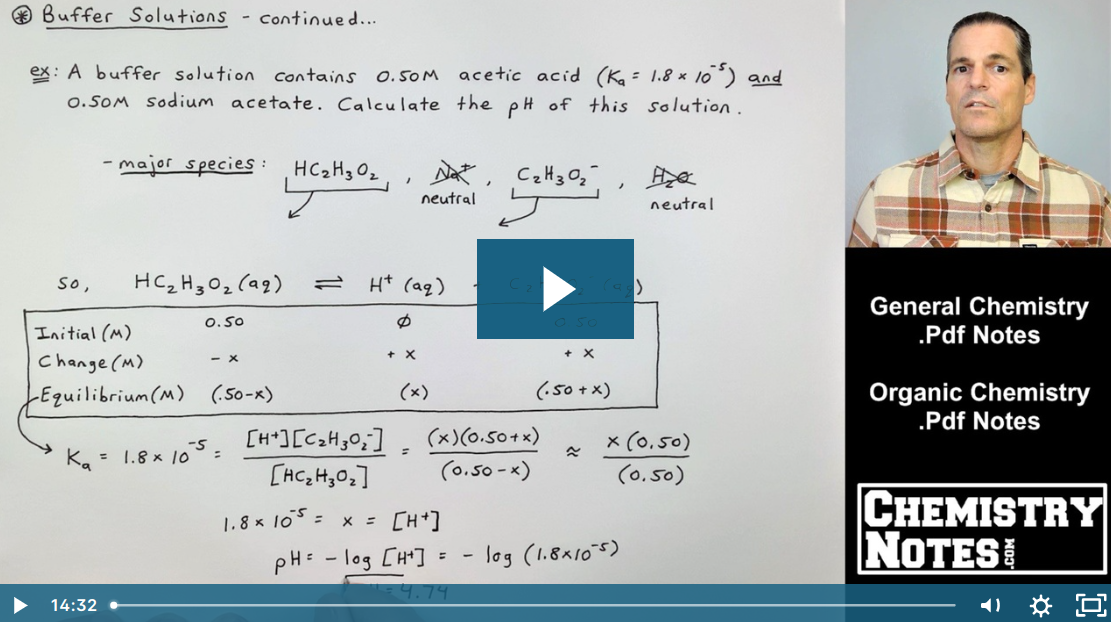
PPT – Calculate the pH of the 0.30 M NH3/0.36 M NH4Cl buffer system. What is the pH after the addition of 20.0 mL of 0.050 M NaOH to 80.0 mL of

SOLVED: Calculate the pH of a buffer solution made from 0.30 M hydrofluoric acid and 0.70 M sodium fluoride after the addition of 0.080 mol ofNaOHto ofthis solution Assume change in volume

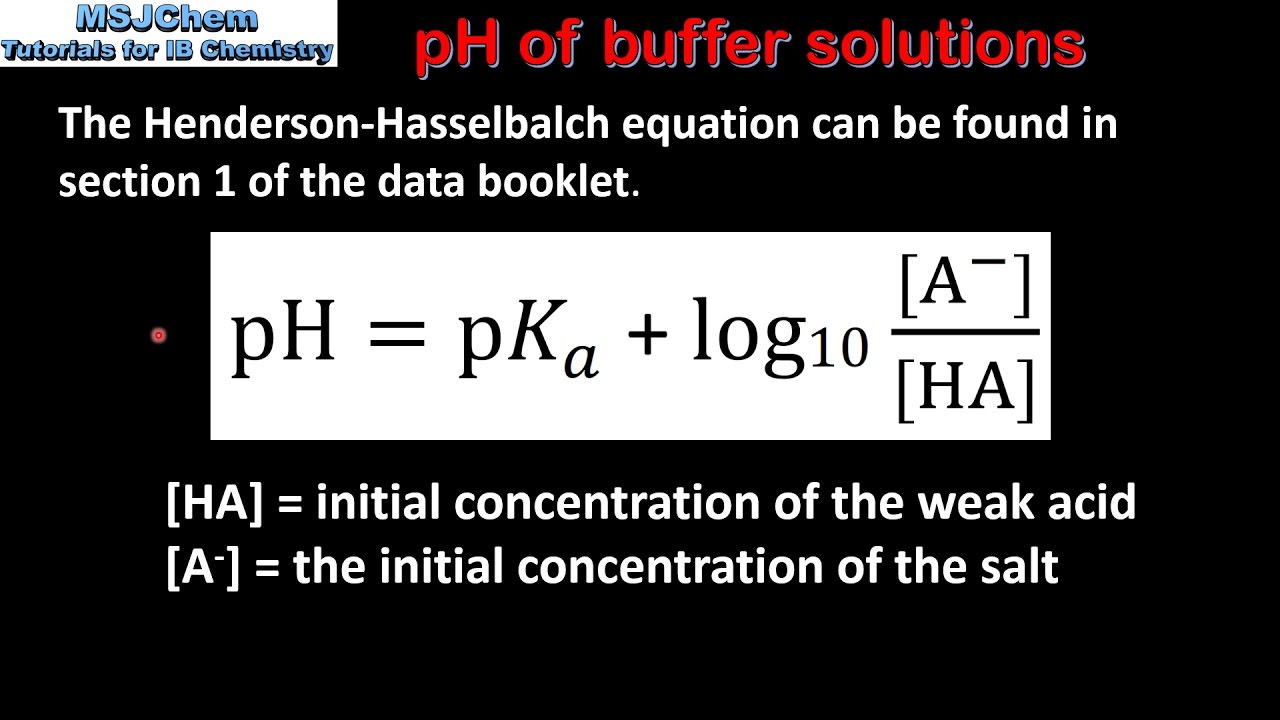
Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com

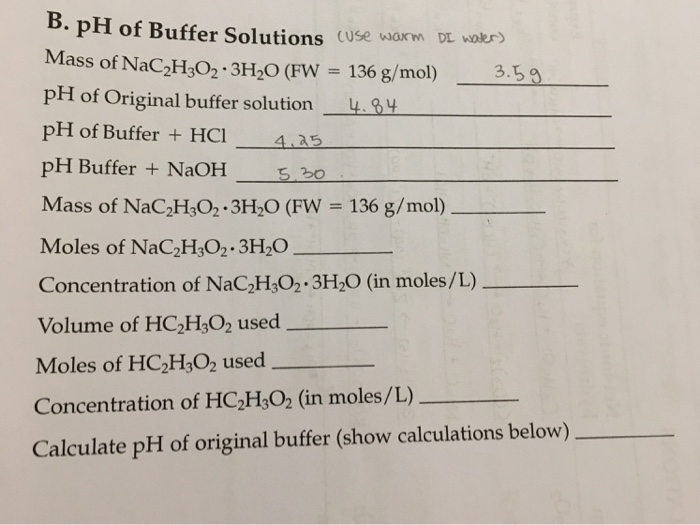
SOLVED: B. pH of Buffer Solutions '9. 50 9 Mass of NaCzH;Oz * 3HzO (FW 1365 g/mol) 41 pH of Original buffer solution of Buffer + HCI 4S pH pH Buffer +

Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com
![Calculate the pH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 mL of an aqueous solution containing 150 mL of 1M HCl . Ka for: HCO3 = 5.63 × 10^-11 [ log 133150 = - 0.05 ] Calculate the pH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 mL of an aqueous solution containing 150 mL of 1M HCl . Ka for: HCO3 = 5.63 × 10^-11 [ log 133150 = - 0.05 ]](https://dwes9vv9u0550.cloudfront.net/images/6404140/adc2d8b1-954e-4b3c-b9c4-733c3311ce8f.jpg)
Calculate the pH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 mL of an aqueous solution containing 150 mL of 1M HCl . Ka for: HCO3 = 5.63 × 10^-11 [ log 133150 = - 0.05 ]