Use of Biomarkers to Guide Decisions on Systemic Therapy for Women With Metastatic Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline | Journal of Clinical Oncology
Protocol for the Examination of Resection Specimens From Patients With Invasive Carcinoma of the Breast

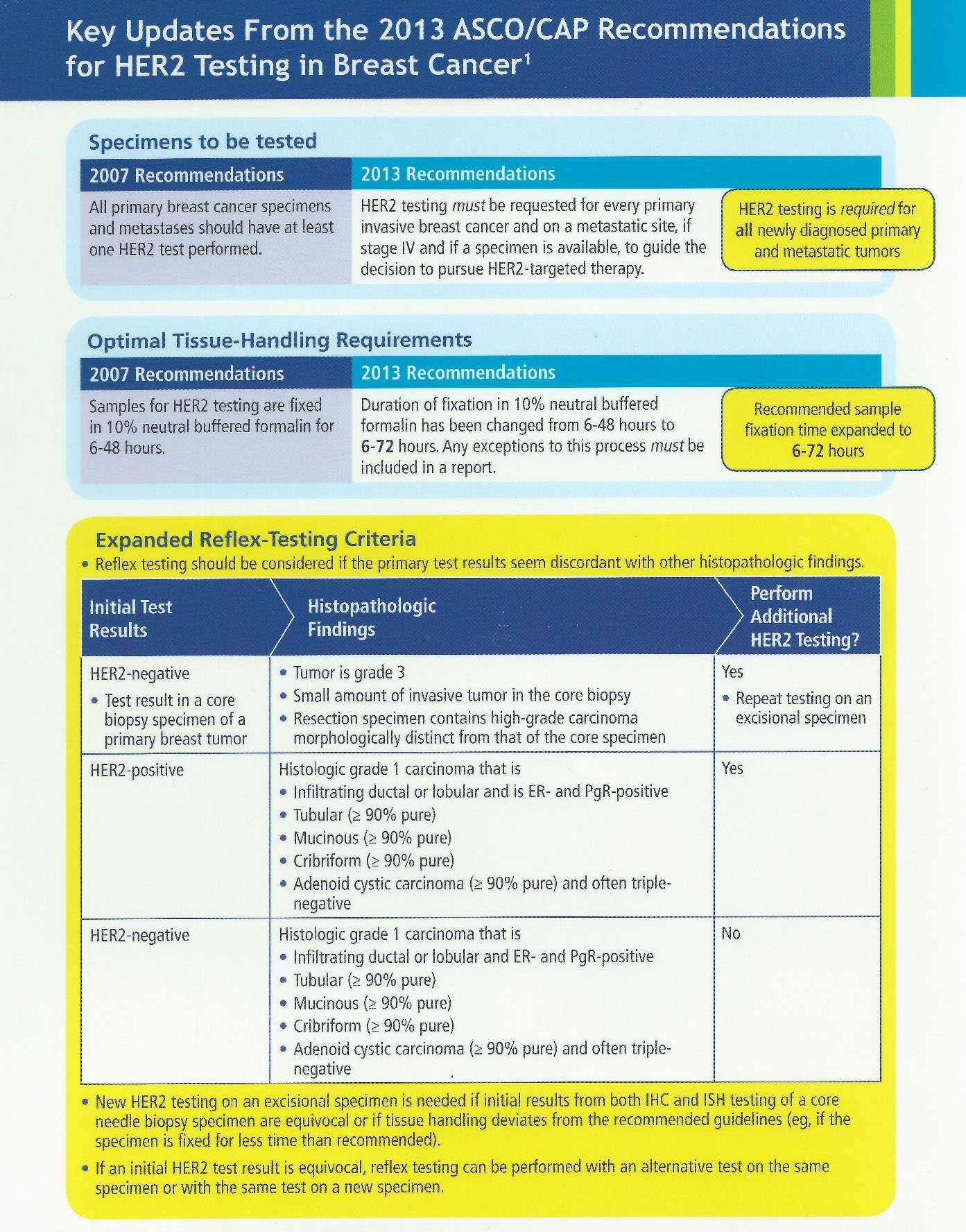
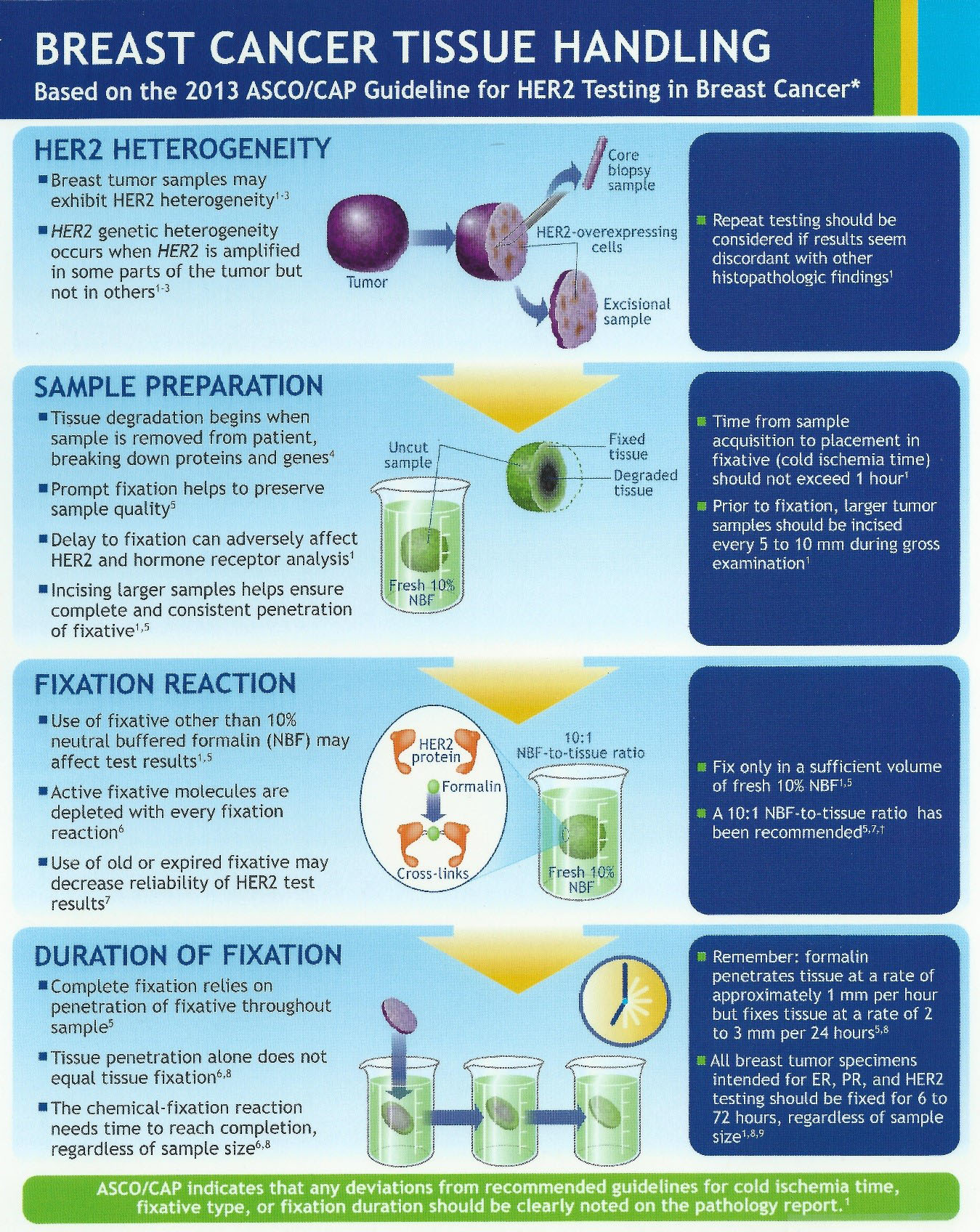
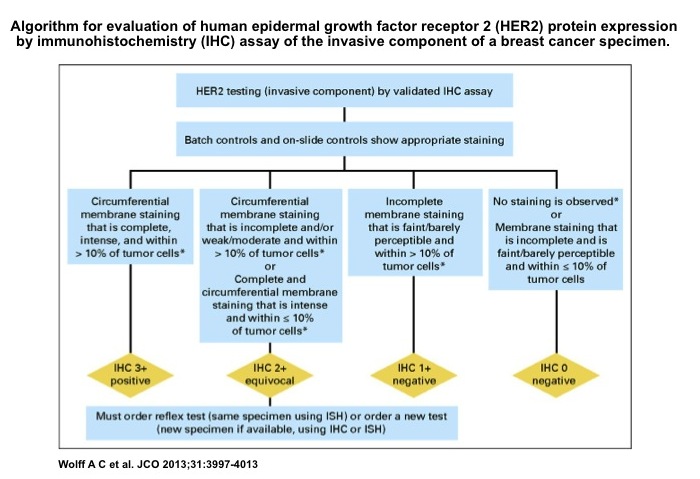
Impact of 2013 ASCO/CAP guidelines on HER2 determination of invasive breast cancer: A single institution experience using frontline dual-color FISH - ScienceDirect
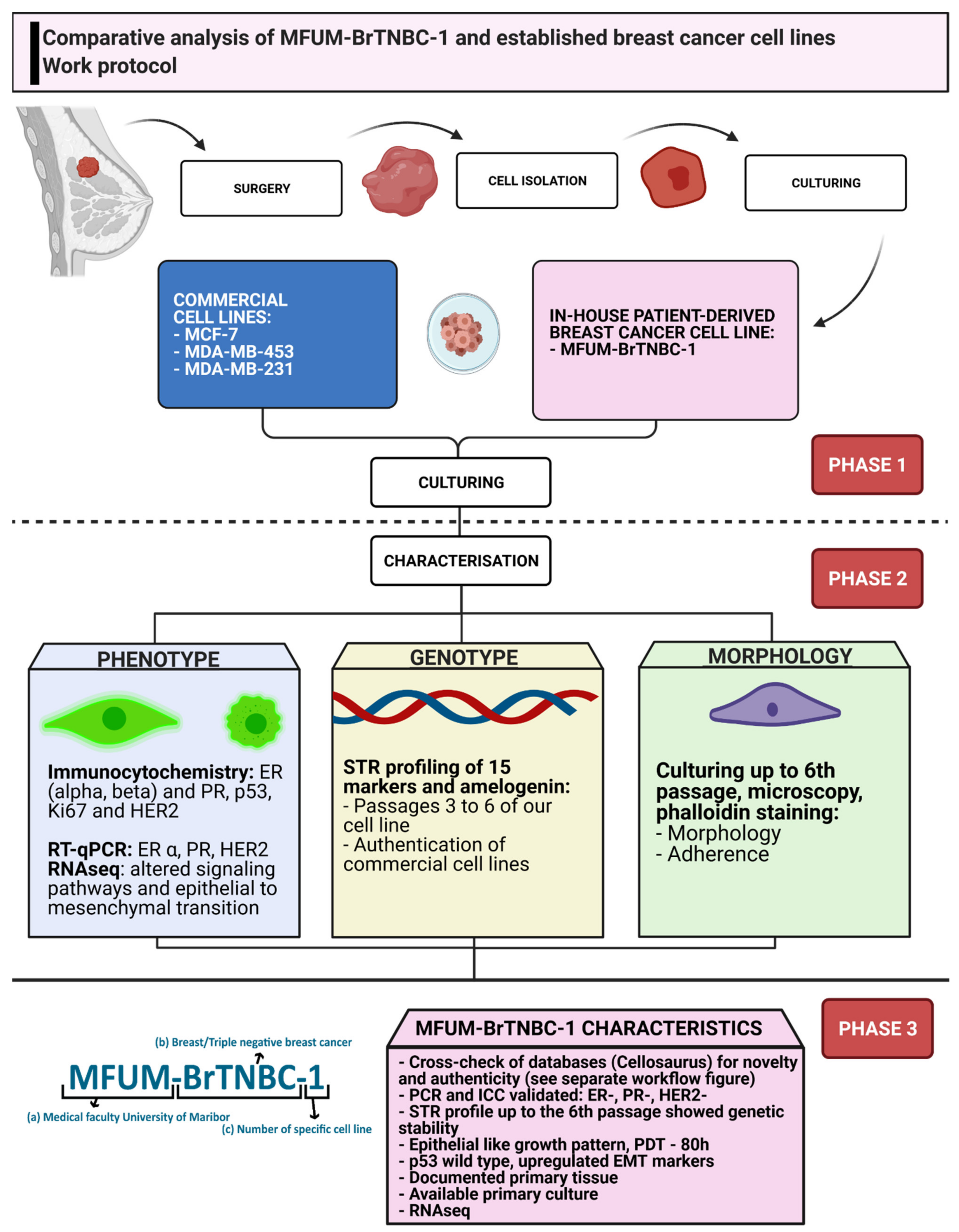
Cells | Free Full-Text | MFUM-BrTNBC-1, a Newly Established Patient-Derived Triple-Negative Breast Cancer Cell Line: Molecular Characterisation, Genetic Stability, and Comprehensive Comparison with Commercial Breast Cancer Cell Lines | HTML

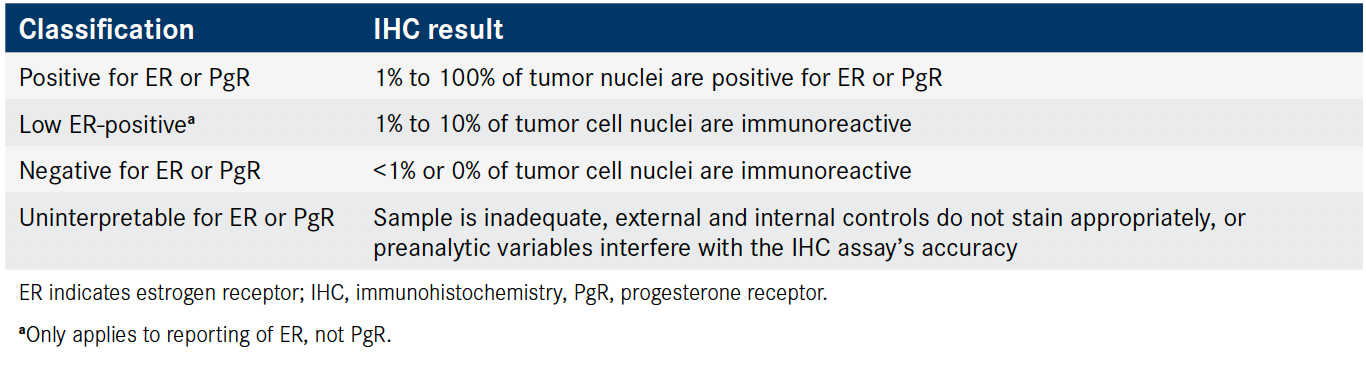
Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update | Journal of Clinical Oncology
Protocol for the Examination of Resection Specimens from Patients with Invasive Carcinoma of the Breast
Protocol for the Examination of Biopsy Specimens From Patients With Invasive Carcinoma of the Breast

PDF) American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer

Redefining breast cancer subtypes to guide treatment prioritization and maximize response: Predictive biomarkers across 10 cancer therapies - ScienceDirect
Protocol for the Examination of Resection Specimens from Patients with Ductal Carcinoma In Situ (DCIS) of the Breast
![PDF] HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation | Semantic Scholar PDF] HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b66331181b2eafa14b6e478dcc7fced0c7917993/3-Table1-1.png)
PDF] HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation | Semantic Scholar
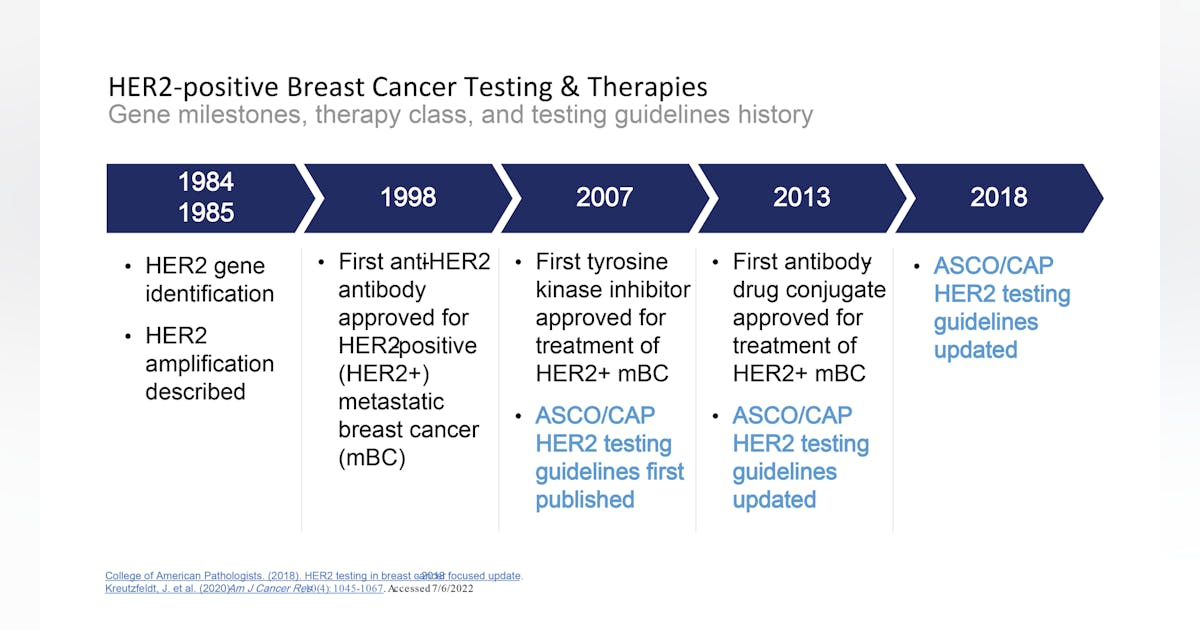
Breast cancer biomarkers, and a new clinical category for HER2 expression | Medical Laboratory Observer
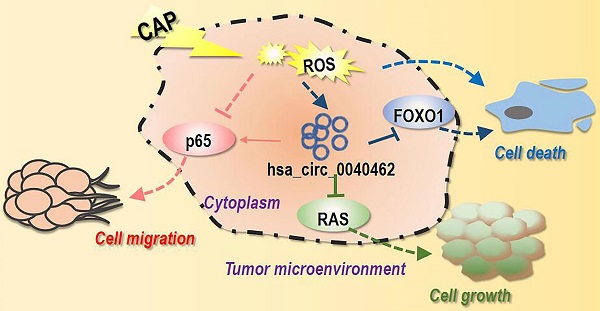
Hsa_circRNA_0040462: a sensor of cells' response to CAP treatment with double-edged roles on breast cancer malignancy