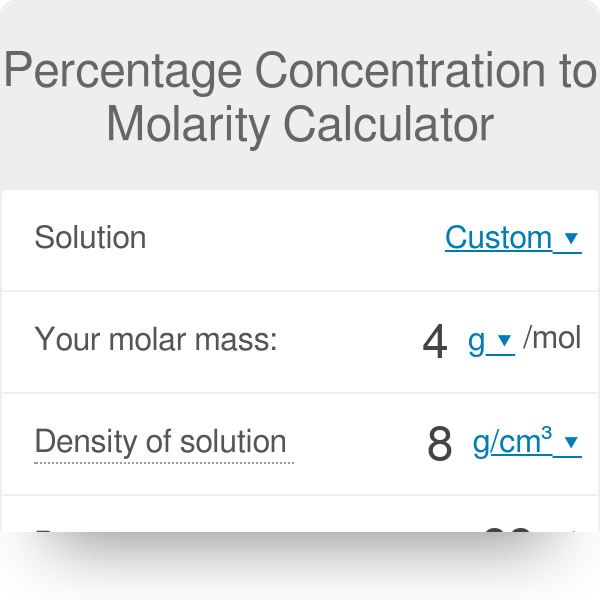
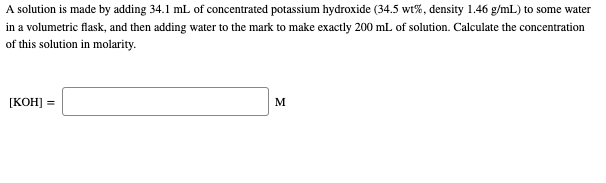
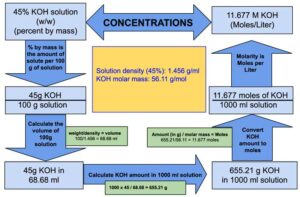
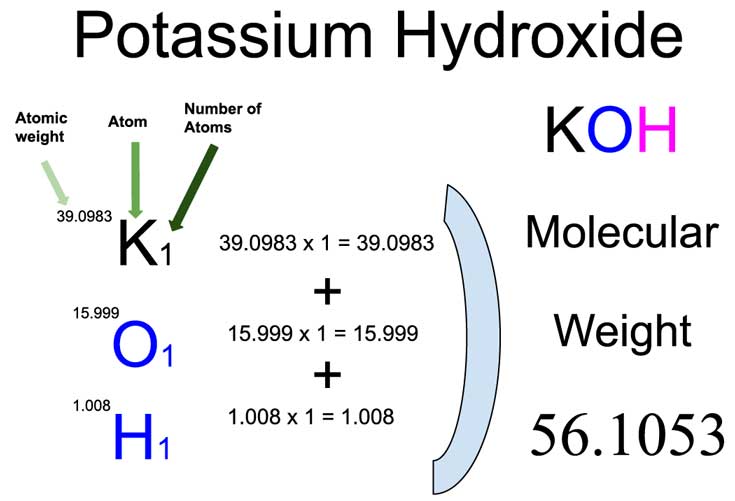
Calculate the molarity of `KOH` in solution prepared by dissolving `5.6 g` in enough water to - YouTube

SOLVED: 114 Write the balanced chemical equation for the reaction of malonic acid with pO- tassium hydroxide: Calculate the molar concentration of the malonic acid if 38.17 mL of 0.315 M potassium

A `6.90 M` solution of `KOH` contains 30% by weight of `KOH`. Calculate the density of the solut... - YouTube

A 7.0 M solution of KOH in water contains 28% by mass of KOH. What is density of solution in gm/ml ?
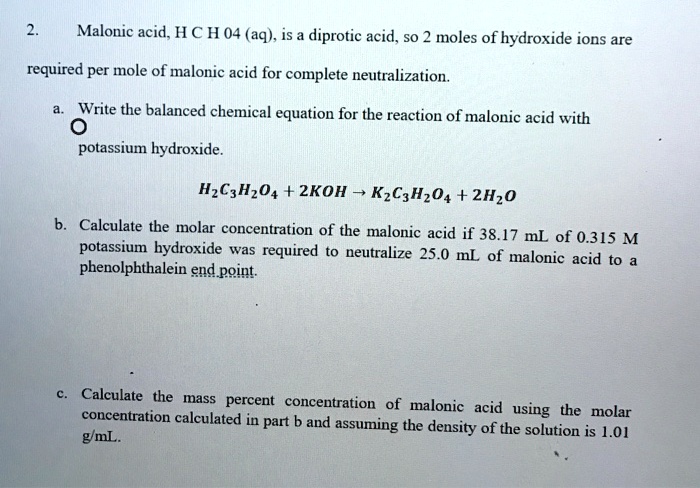
SOLVED: Malonic acid, H C H 04 (aq). is a diprotie acid, So 2 moles of hydroxide ions are required per mole of malonic acid for complete neutralization. Write the balanced chemical

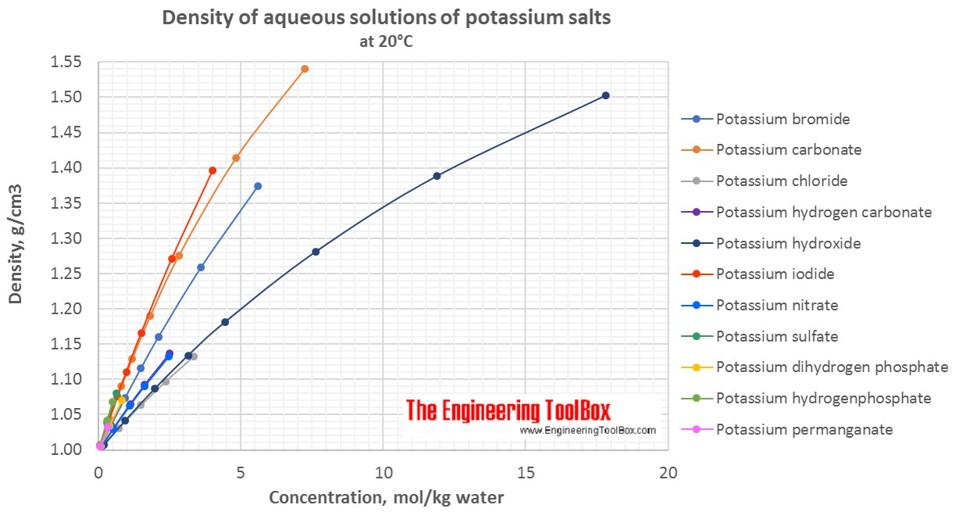
Effect of potassium hydroxide concentration in the electrolyte on the... | Download Scientific Diagram

SOLVED: equation for the reaction of malonic acid with po: Write the balanced chemical . HEUME hrdroxide Calculate the molar concentration of the malonic acid if 38.17 mL of 0.315 M potassium
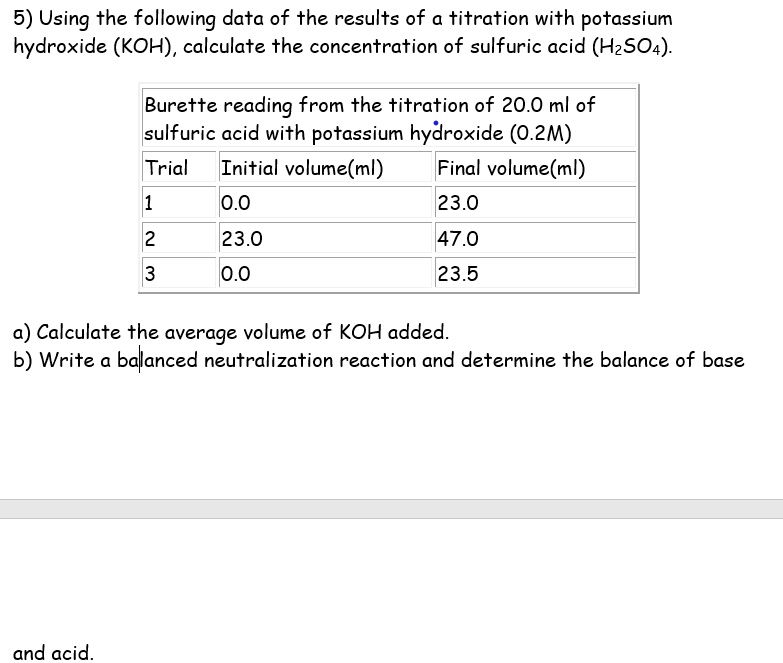
SOLVED: 5) Using the following data of the results of a titration with potassium hydroxide (KOH), calculate the concentration of sulfuric acid (HzSO4) Burette reading from the titration of 20.0 ml of

36cm<sup>3</sup> of a solution of potassium hydroxide requires 25cm<sup>3</sup> of 0.5M sulphuric acid to neutralize it.Calculate the concentration of alkali in g/dm<sup>3</sup>