A theoretical analysis on enthalpy of vaporization: Temperature-dependence and singularity at the critical state - ScienceDirect

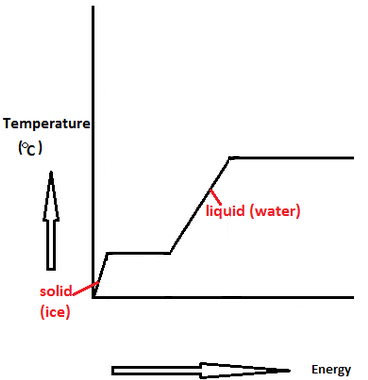
The enthalpy of vaporization of water at 100^o C is 40.63 KJ mol^-1 . The value Δ E for this process would be:
Study suggests that increased enthalpies of vaporization in ethanol/gasoline mixtures could account for increased PM emissions from GDI engines fueled with ethanol blends - Green Car Congress

SOLVED: 7 The enthalpy of vaporization of water at 100.0-C is 40.68 kJ mol. What is the entropy change in the surroundings when one mole of water vapor condenses at 100.0 %C

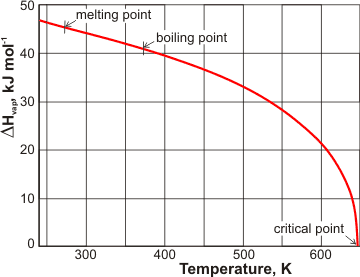
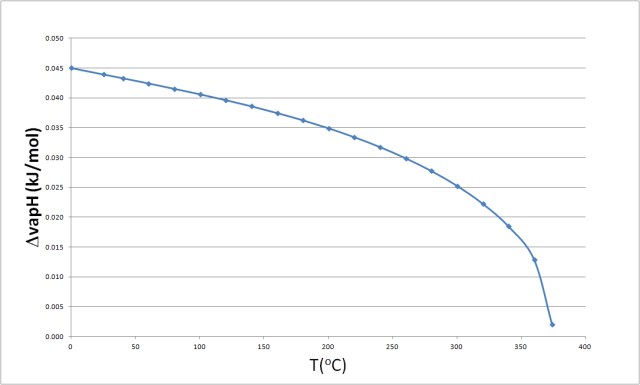
Enthalpy of vaporization of water: (—) Reference fundamental equation... | Download Scientific Diagram

SOLVED: The enthalpy of vaporization of water is 44 kJ/mol. How much heat would be released if 100 grams of water vapor condenses to liquid water? The answer is 244 kj but

Molar enthalpy of vaporization of a liquid is 2.6 kJ. If boiling point of this liquid is `160^(@)c`, - YouTube

thermodynamics - Does adding salt to water decrease the latent heat of vaporization? - Physics Stack Exchange

Standard enthalpy of vapourisation ΔvapH^ (0) for water at 100 is 40.66 kJmol^-1 . The internal energy of vapourisation of water at 100 (in kJmol^-1 ) is: