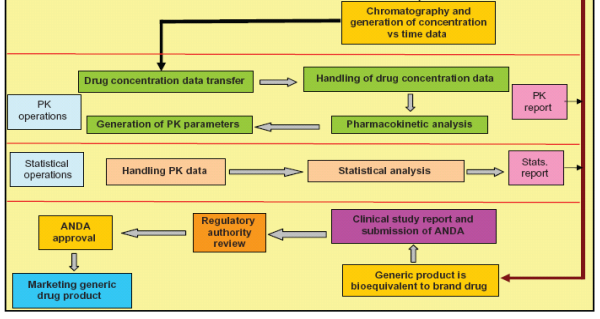
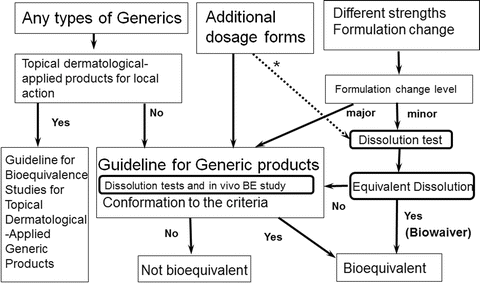
Study of regulatory requirements for the conduct of bioequivalence studies in US, Europe, Canada, India, ASEAN and SADC countrie

Pharmacogenetic perspectives in improving pharmacokinetic profiles for efficient bioequivalence trials with highly variable drugs: a review | International Journal of Pharmacokinetics

Bioequivalence of topical generic products. Part 2. Paving the way to a tailored regulatory system - ScienceDirect

GENERIC DRUGS: Guidelines for bioequivalence studies: Vishwakarma, Pushpendra Kumar: 9783639343779: Amazon.com: Books

GENERIC DRUGS: Guidelines for bioequivalence studies: Vishwakarma, Pushpendra Kumar: 9783639343779: Amazon.com: Books

PDF) International Guidelines for Bioequivalence of Systemically Available Orally Administered Generic Drug Products: A Survey of Similarities and Differences

Current regulatory scenario and alternative surrogate methods to establish bioequivalence of topical generic products - ScienceDirect
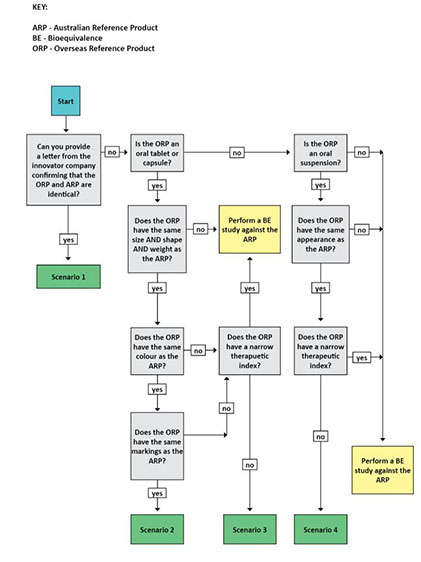
15.6 Choice of the reference product for bioequivalence of generic medicines | Therapeutic Goods Administration (TGA)
PPT - Bioequivalence studies: Regulatory Requirements on Conduct & Documentation of BE. PowerPoint Presentation - ID:5123884
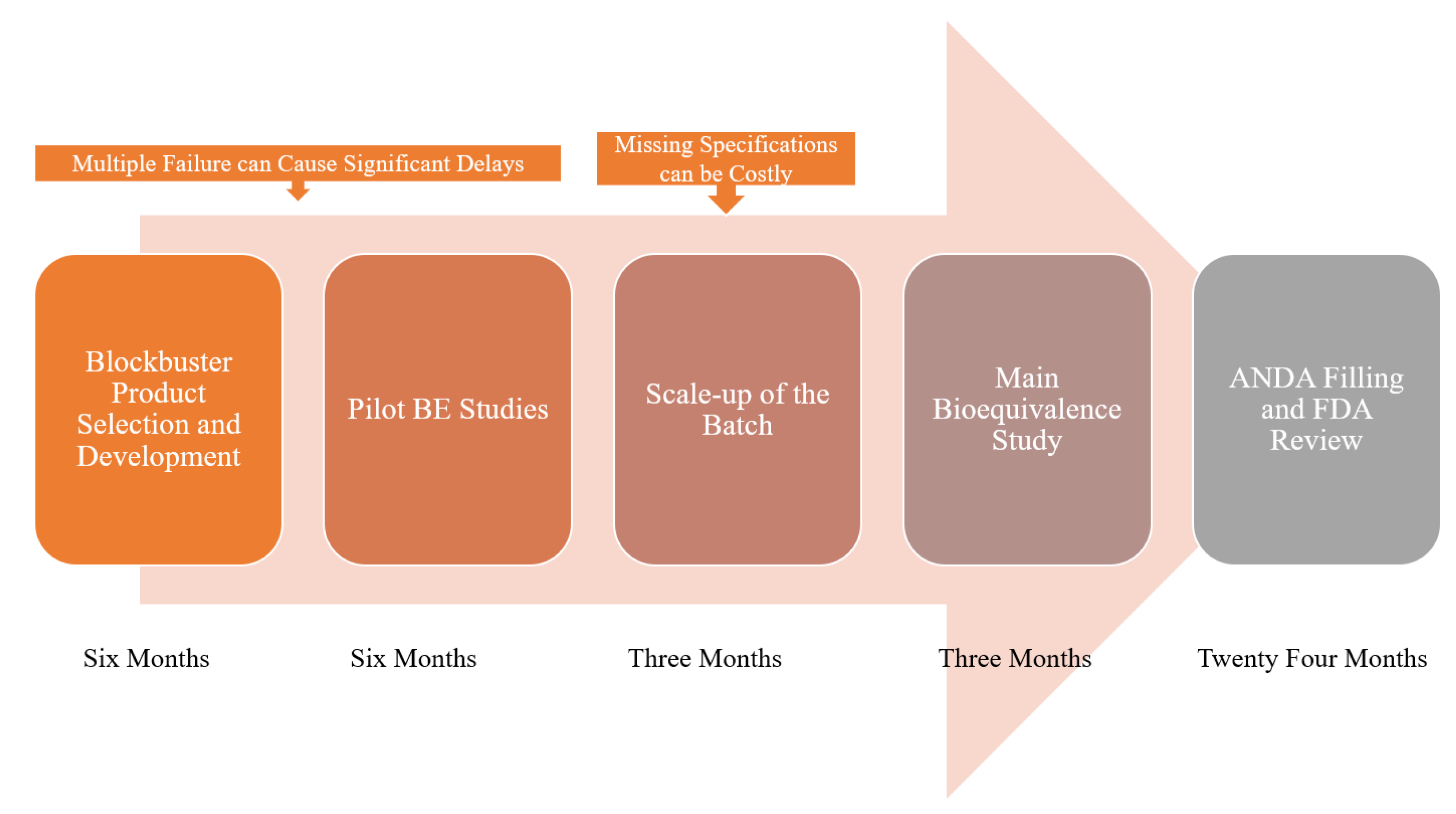
Pharmaceutics | Free Full-Text | In Vitro Dissolution and in Silico Modeling Shortcuts in Bioequivalence Testing | HTML