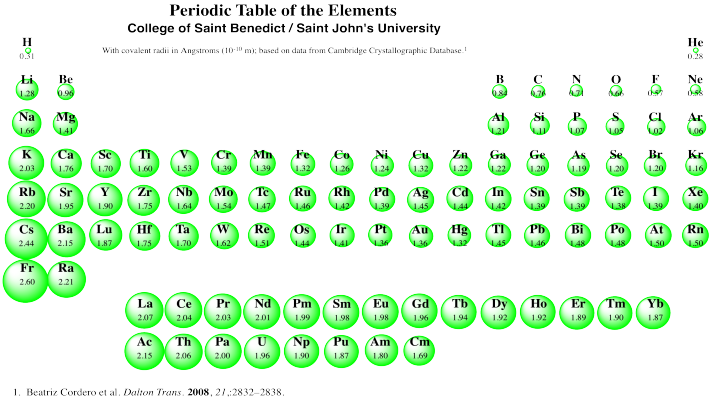
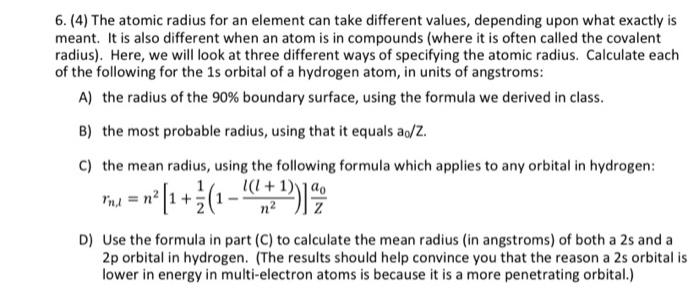
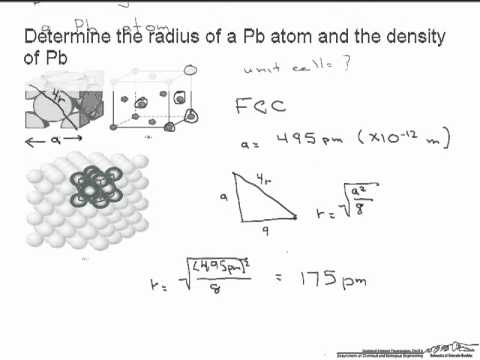
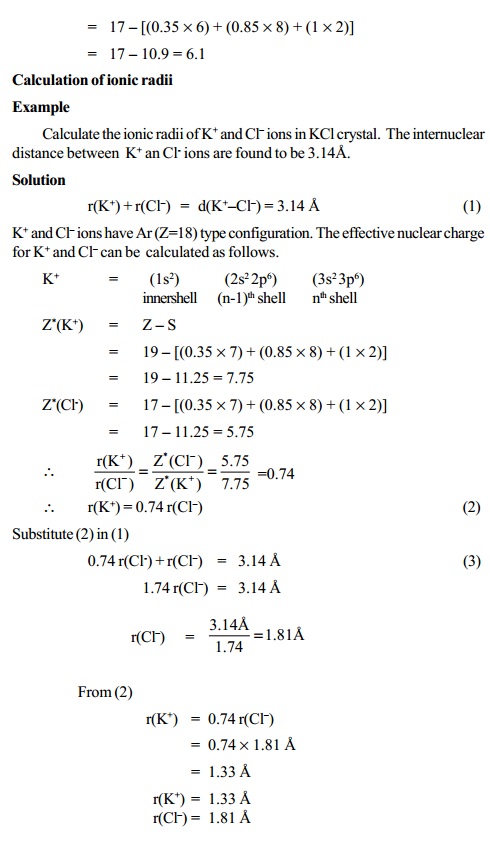
Niobium has a density of 8.57 g/cm3 and crystallizes with the body-centered cubic unit cell. Calculate the radius of a niobium atom - Chemistry Stack Exchange

Ionic Radius Trends, Basic Introduction, Periodic Table, Sizes of Isoelectric Ions, Chemistry - YouTube

Atomic radius of `Li is 1.23 Å` and ionic radius of `Li^(+)` is `0.76 Å`. Calculate the percentage - YouTube