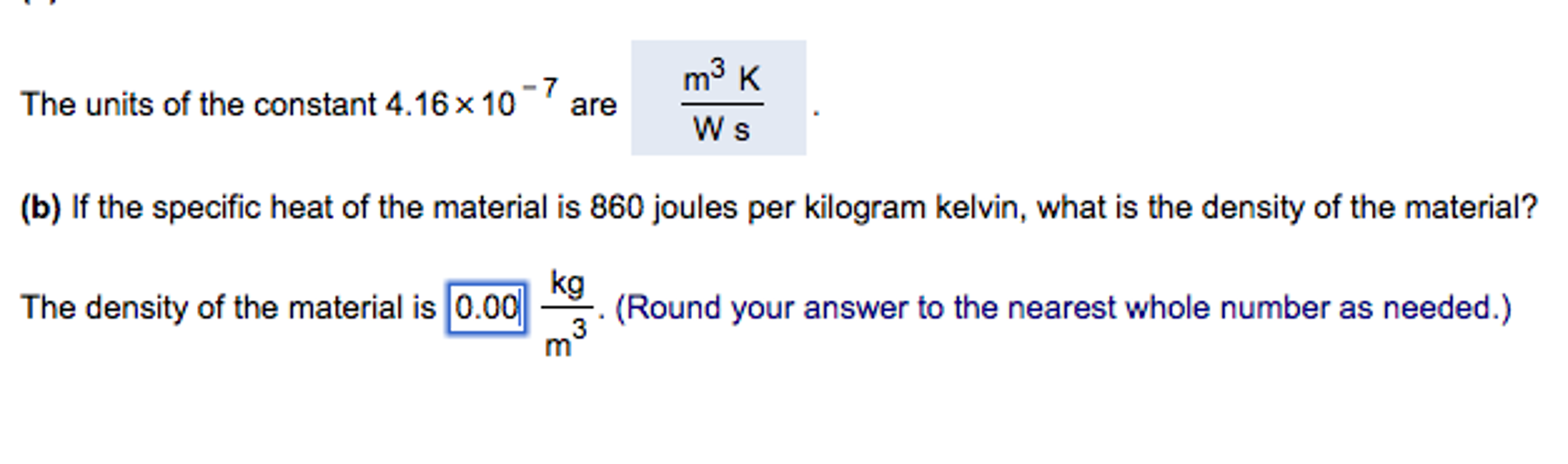
Solved] Calculate the temperature in "C of a gas in when its volume is 8.7 L, if the same sample of gas has a volume of 4.0 L at 60 C. Answer:...

John Carlos Baez on Twitter: "Entropy is missing information. But we can measure changes in entropy by doing experiments. So if we assume hydrogen has no entropy at absolute zero, we can

Understanding the SI Units (meters, seconds, kg, kelvin, coulomb, candela) MCAT Physics Chemistry - YouTube
Specific heat of hydrogen at constant pressure is 30 joule per Kelvin per mol. If unit of length changed to 50 cm, unit of time changes to 1/4 sec and unit of

SOLVED: The Ideal Gas Constant has units of Joule * (mole)-Ix (Kelvin)-1. In SI base units this is: kg m2 s-2 K-1 . mol-1 kg m . K-1 mol-1 . s-1 kg