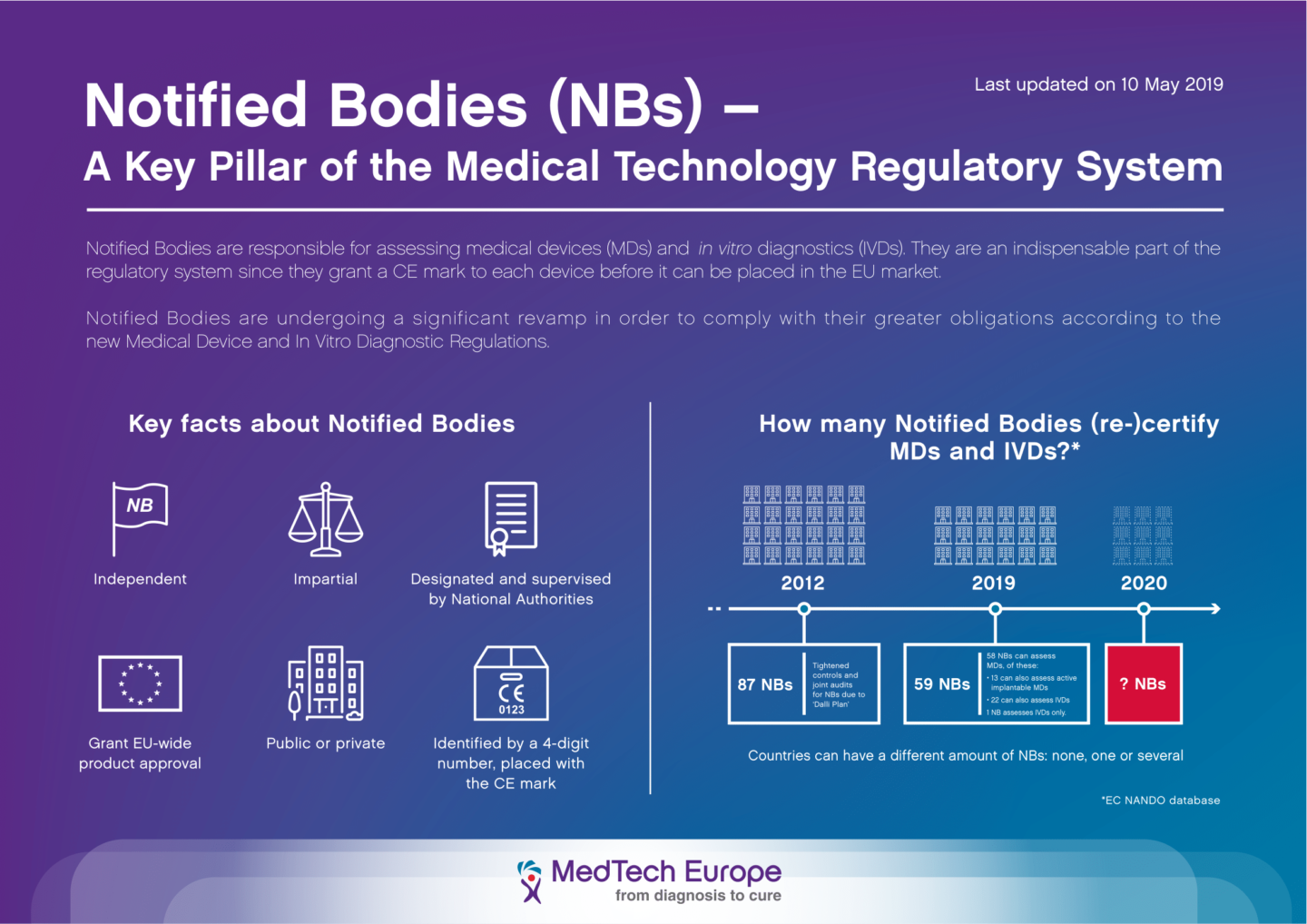
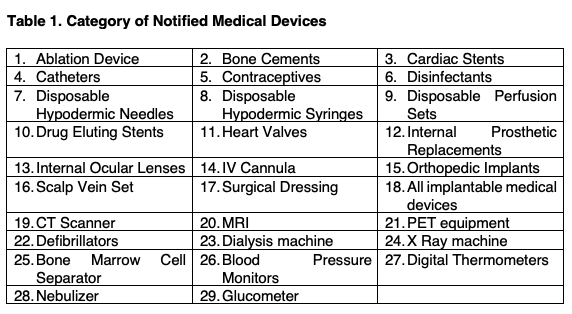
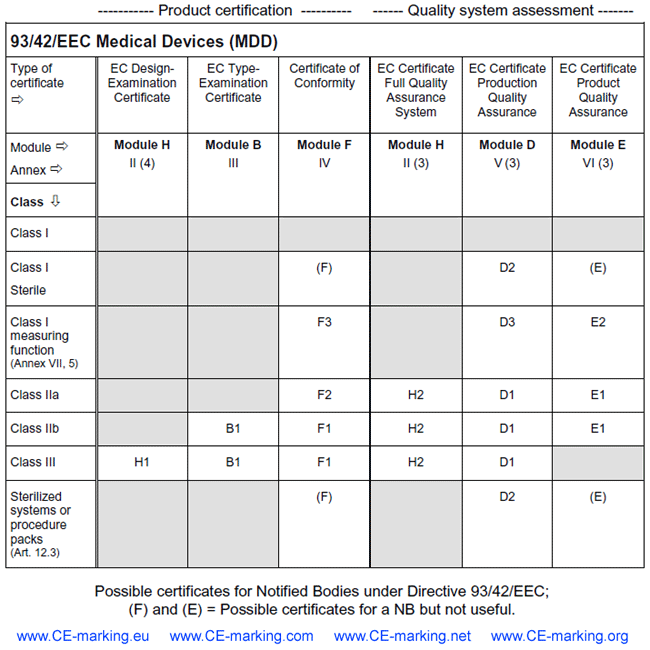
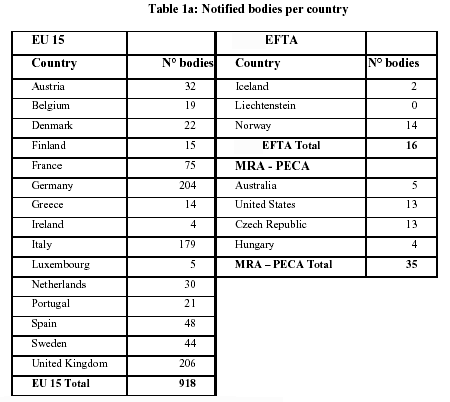
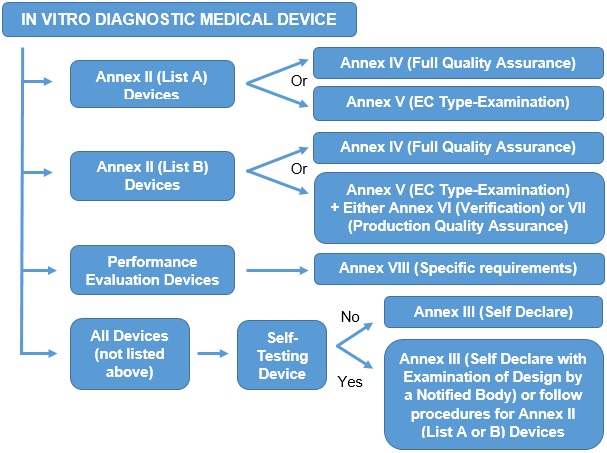
Availability and capacity of notified bodies to carry out conformity assessments for COVID-19 related medical devices and in vit

Approval of artificial intelligence and machine learning-based medical devices in the USA and Europe (2015–20): a comparative analysis - The Lancet Digital Health



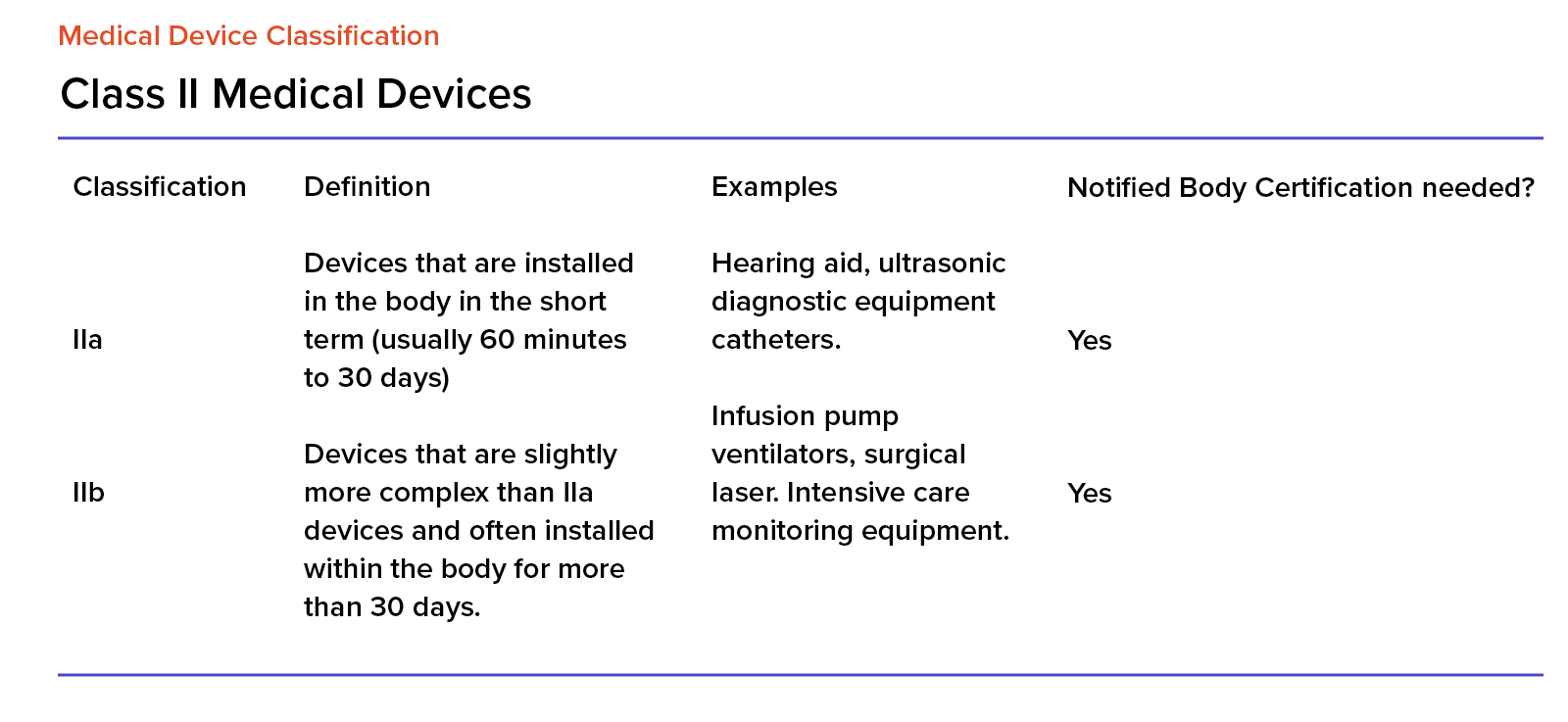

/tuv-rheinland-ivdr-visual-1-en_core_1_x.png)