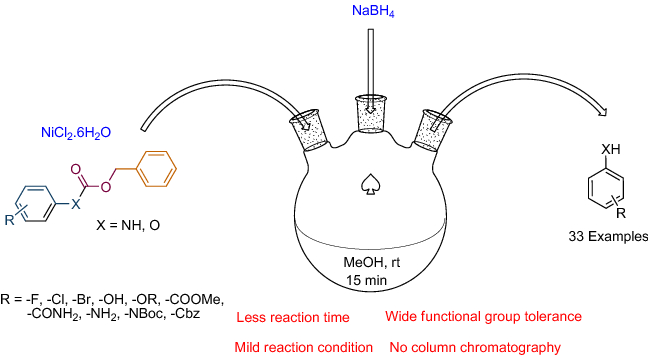
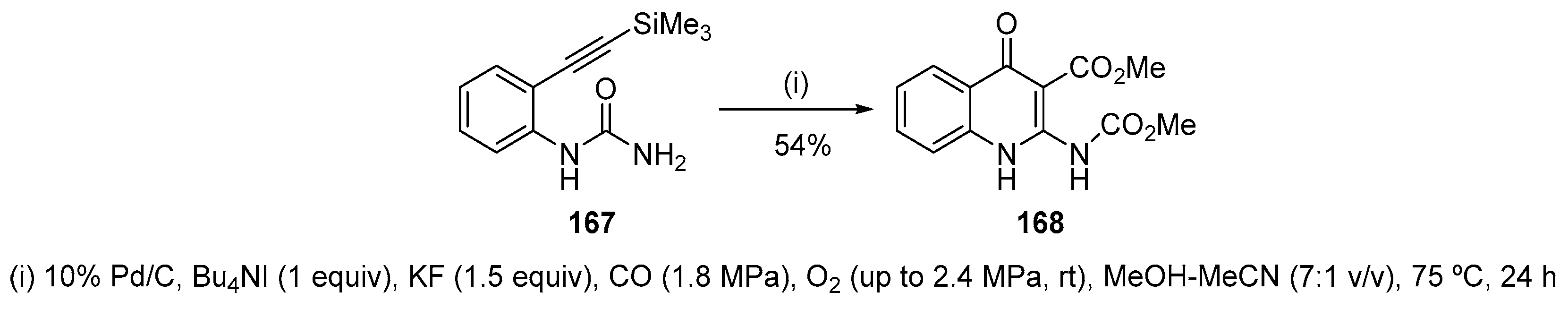
Mild Palladium‐Catalyzed Cyanation of Unprotected 2‐Iodoglycals in Aqueous Media as Versatile Tool to Access Diverse C2‐Glycoanalogues - Malinowski - 2020 - Advanced Synthesis & Catalysis - Wiley Online Library

Palladium-Catalyzed Synthesis of Dibenzosilepin Derivatives via 1,n- Palladium Migration Coupled with anti-Carbopalladation of Alkyne | Journal of the American Chemical Society

PDF) Palladium-catalysed reactions in solid phase organic synthesis | Johannes Köbberling - Academia.edu

Propargylic and Allenic Carbocycle Synthesis through Palladium-Catalyzed Dearomatization Reaction | The Journal of Organic Chemistry
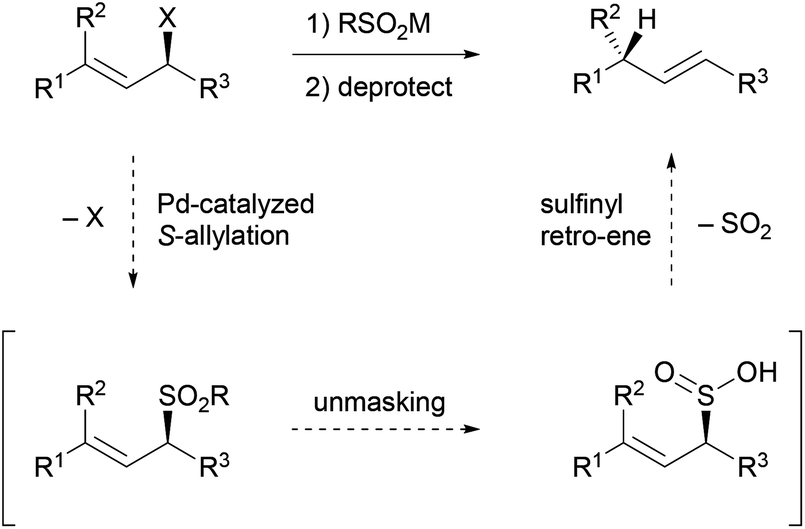
Stereoselective allylic reduction via one-pot palladium-catalyzed allylic sulfonation and sulfinyl retro-ene reactions - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C8QO00233A

Palladium-catalysed carboformylation of alkynes using acid chlorides as a dual carbon monoxide and carbon source | Nature Chemistry
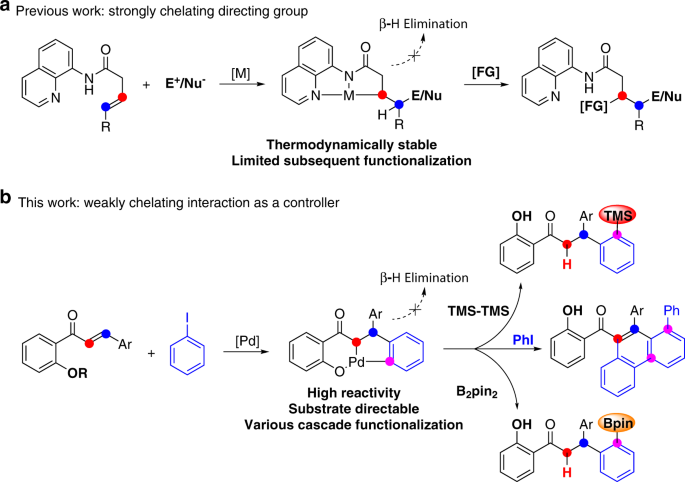
Quadruple C-H activation coupled to hydrofunctionalization and C-H silylation/borylation enabled by weakly coordinated palladium catalyst | Nature Communications

Safe Removal of the Allyl Protecting Groups of Allyl Esters using a Recyclable, Low‐Leaching and Ligand‐Free Palladium Nanoparticle Catalyst - Takagi - 2015 - Advanced Synthesis & Catalysis - Wiley Online Library
Optimised Conditions for the Palladium-Catalyzed Hydrogenolysis of Benzyl and Naphthylmethyl Ethers: Preventing Saturation of Aromatic Protecting Groups | Organic Chemistry | ChemRxiv | Cambridge Open Engage
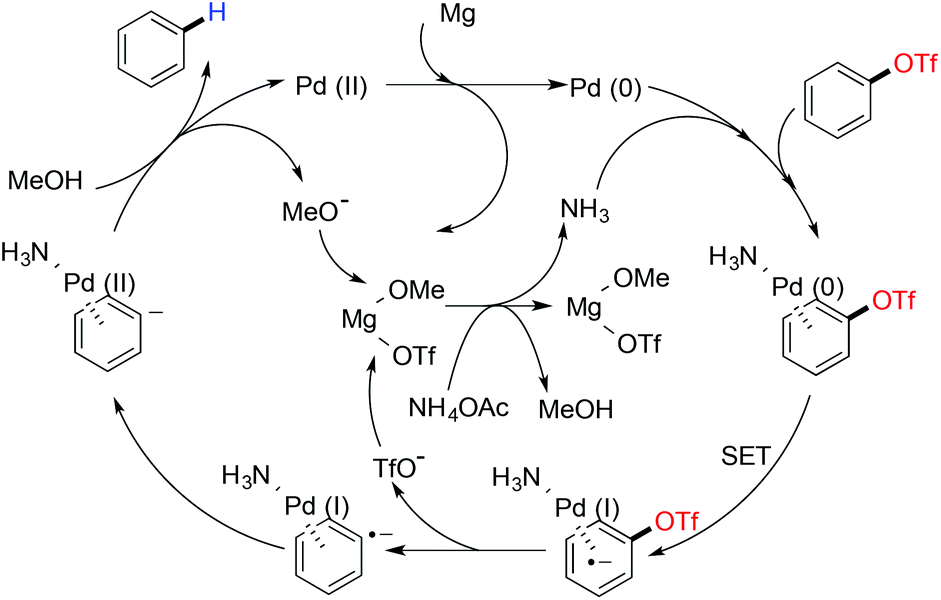
Metal catalyzed defunctionalization reactions - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C5OB01949D
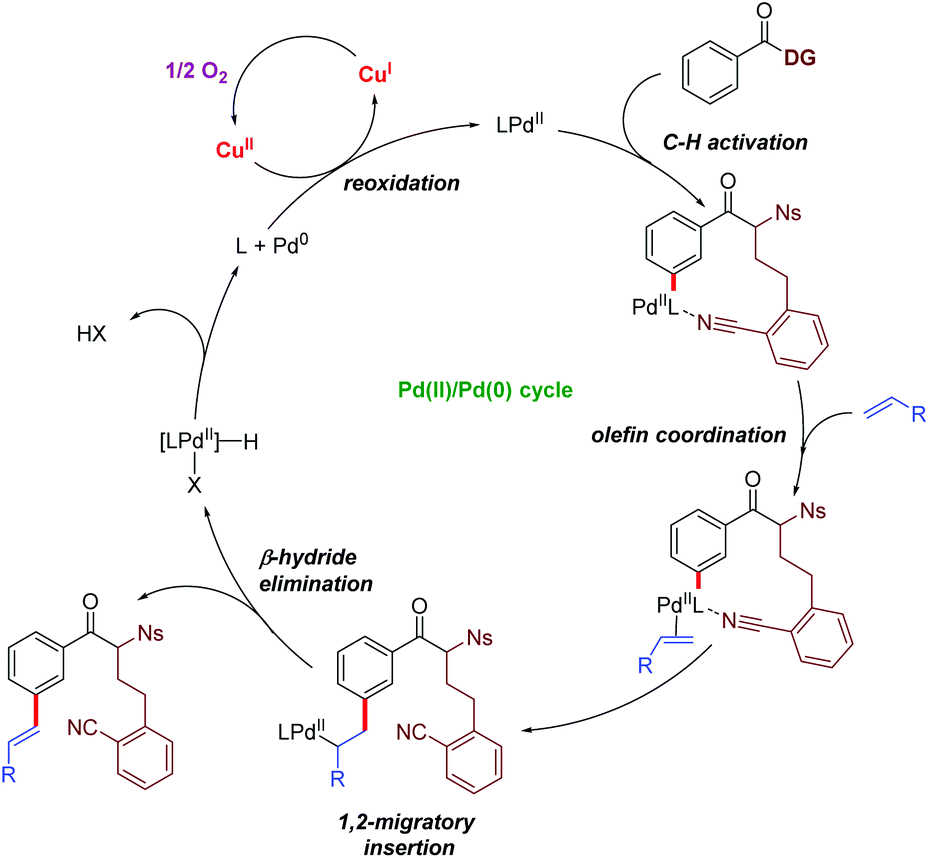
Palladium catalysed meta -C–H functionalization reactions - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C6OB00395H

Intracellular Deprotection Reactions Mediated by Palladium Complexes Equipped with Designed Phosphine Ligands | ACS Catalysis

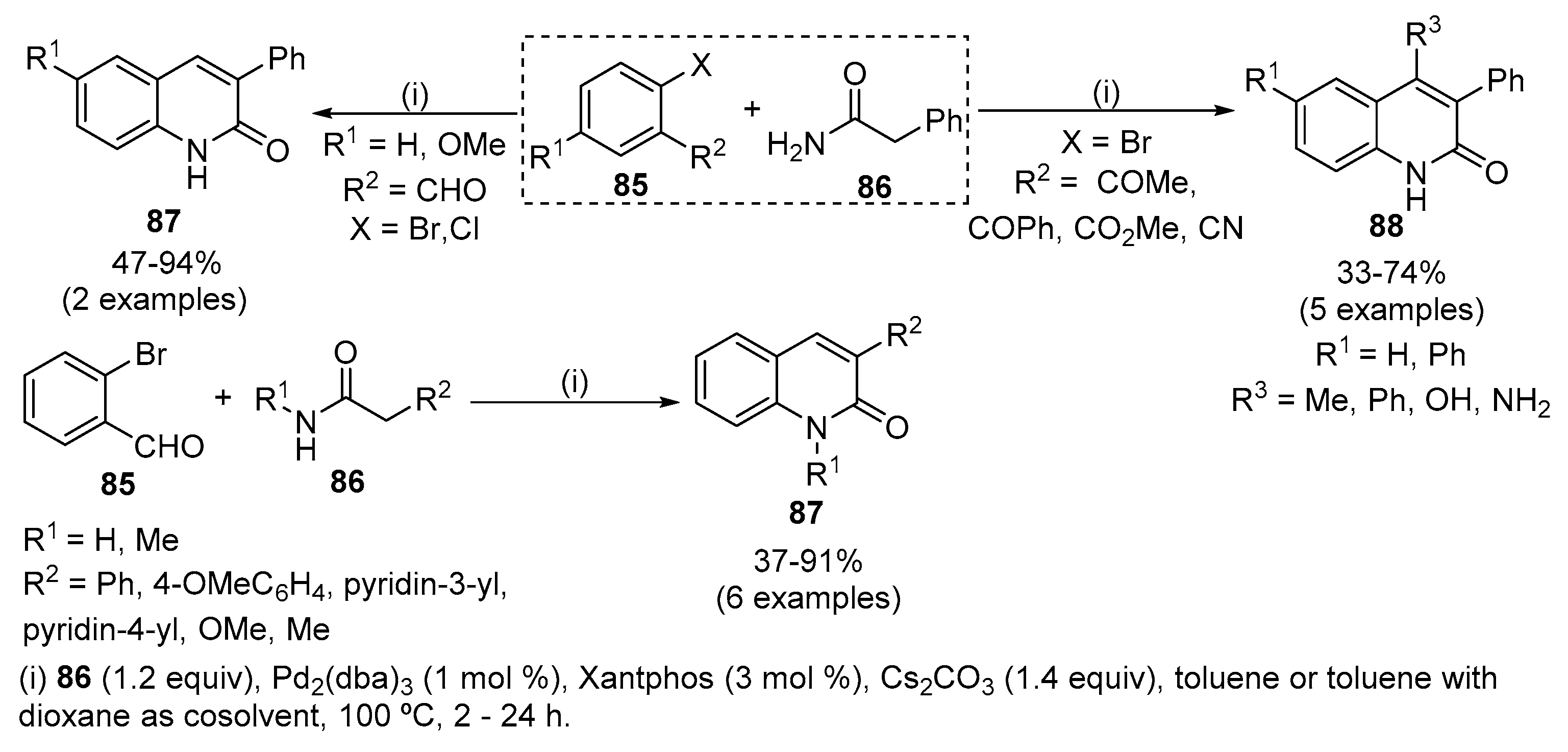
Scope of palladium-catalysed decarboxylative alkylation of lactams.a,... | Download Scientific Diagram

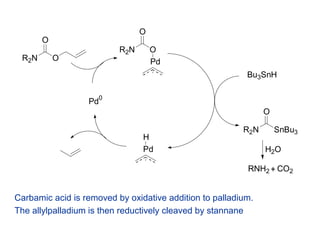
Mechanism of allyl deprotection through catalytic palladium π-allyl... | Download High-Resolution Scientific Diagram

Palladium-triggered deprotection chemistry for protein activation in living cells | Nature Chemistry