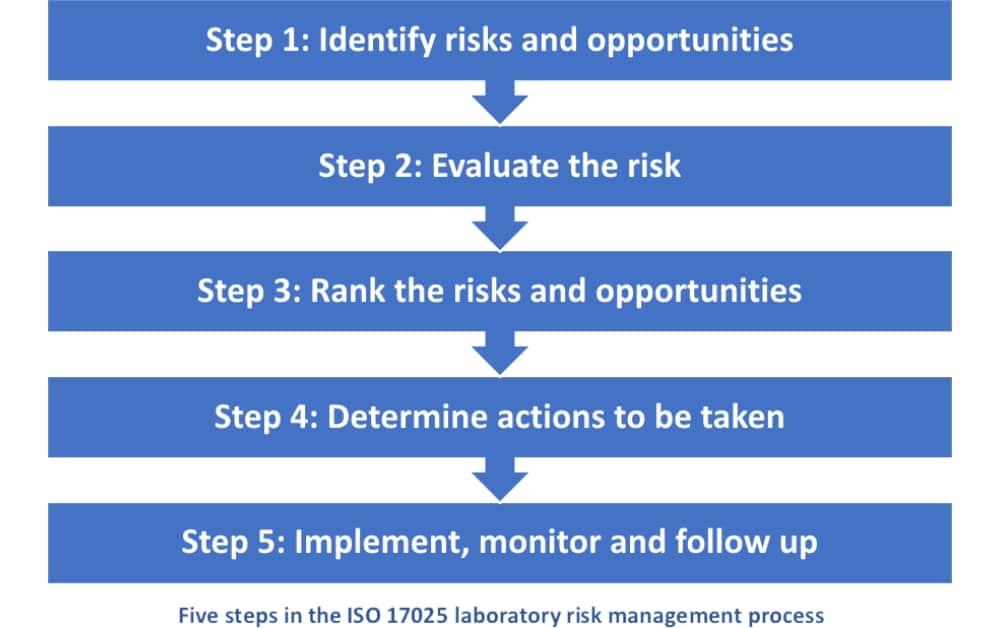
A look at Section 8.5 of ISO/IEC 17025:2017 Presented by: Mike Kramer Calibration/Inspection Program Manager Perry Johnson Labor

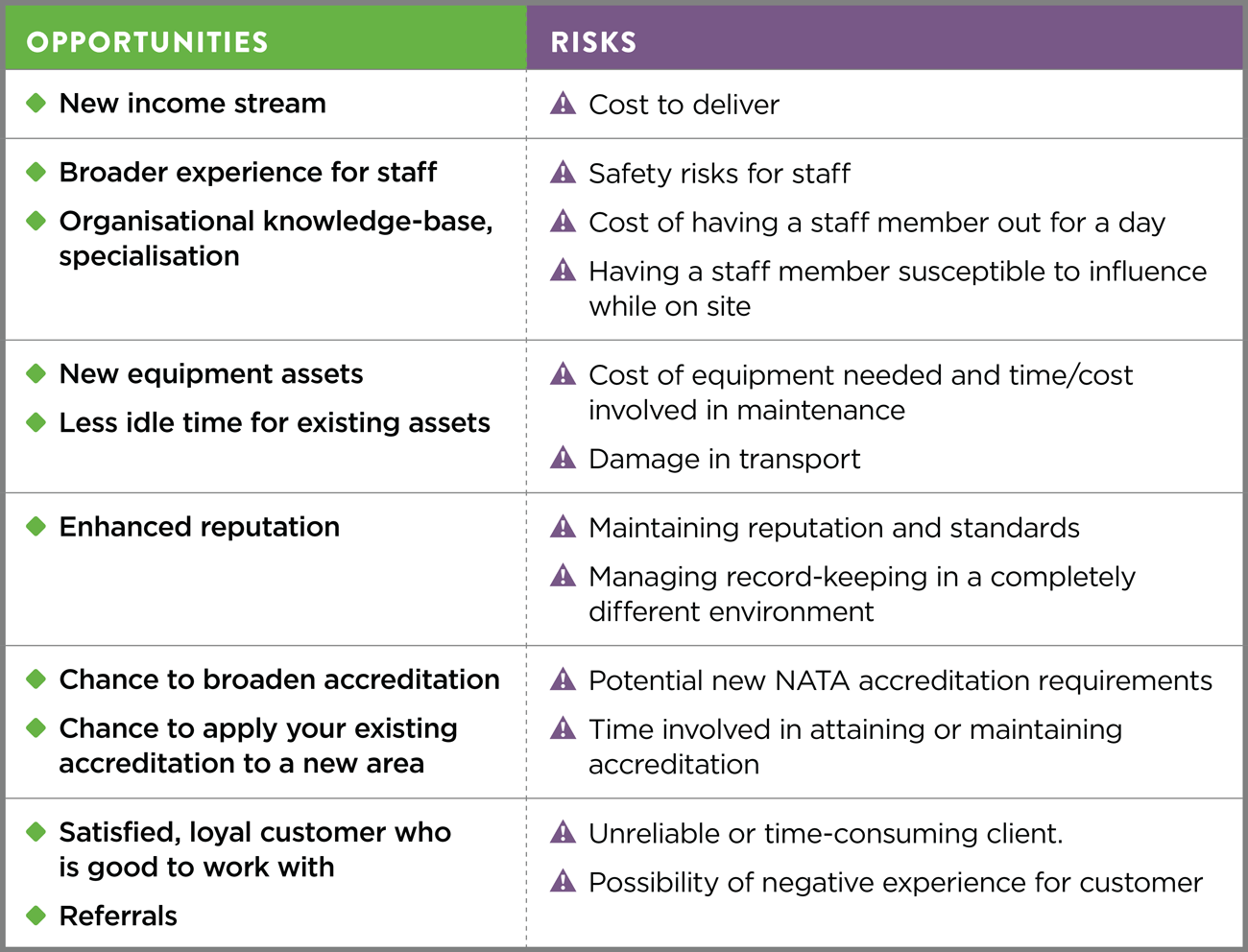
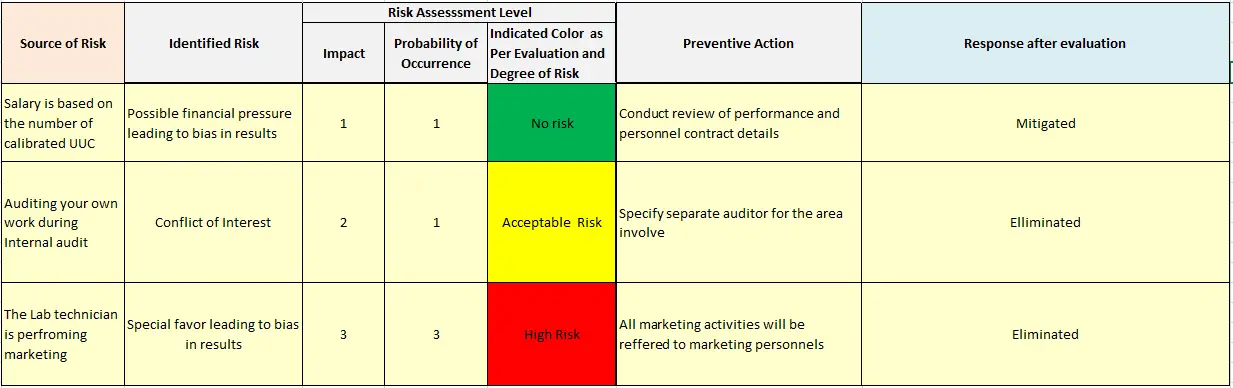
Diagnosis of clause of ISO/IEC 17025:2017, “Actions to address the risk and opportunity (Option A)” ~ Part-1 – QUALiTYViVA
A look at Section 8.5 of ISO/IEC 17025:2017 Presented by: Mike Kramer Calibration/Inspection Program Manager Perry Johnson Labor

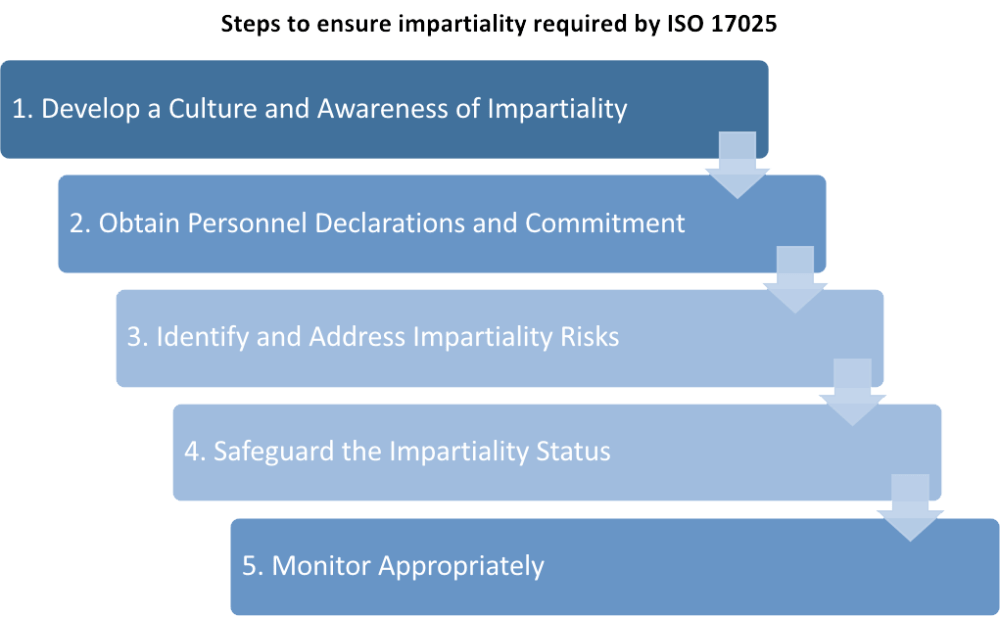
Webinar: It's all about Risk Management: Understanding section 7.7 ISO/IEC 17025:2017 “Ensuring the Validity of Results” | Qualtrax

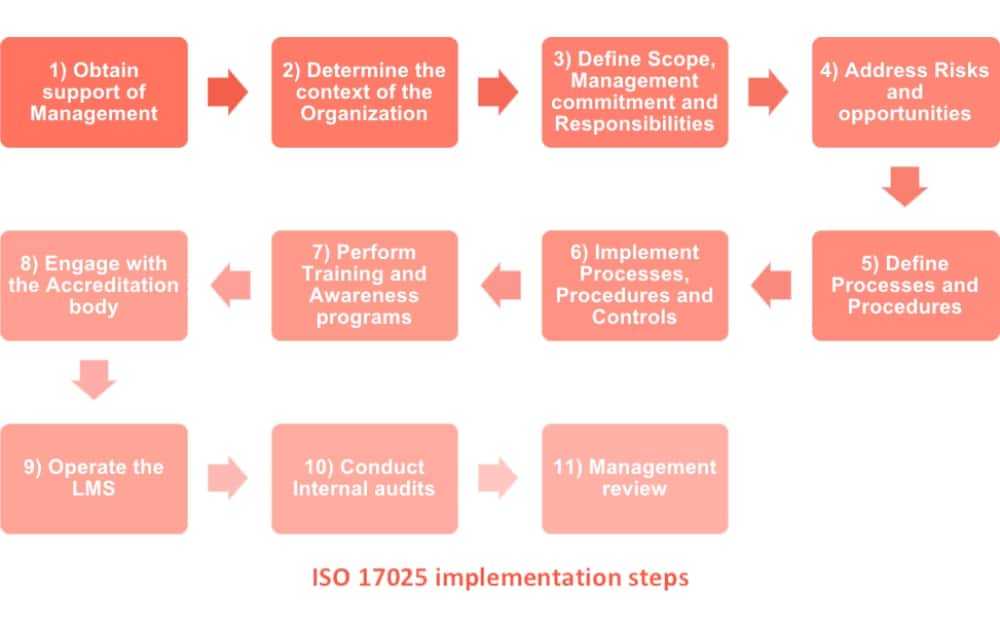
PECB - ISO/IEC 17025:2017 - General Requirements for the Competence of Testing and Calibration Laboratories
A look at Section 8.5 of ISO/IEC 17025:2017 Presented by: Mike Kramer Calibration/Inspection Program Manager Perry Johnson Labor

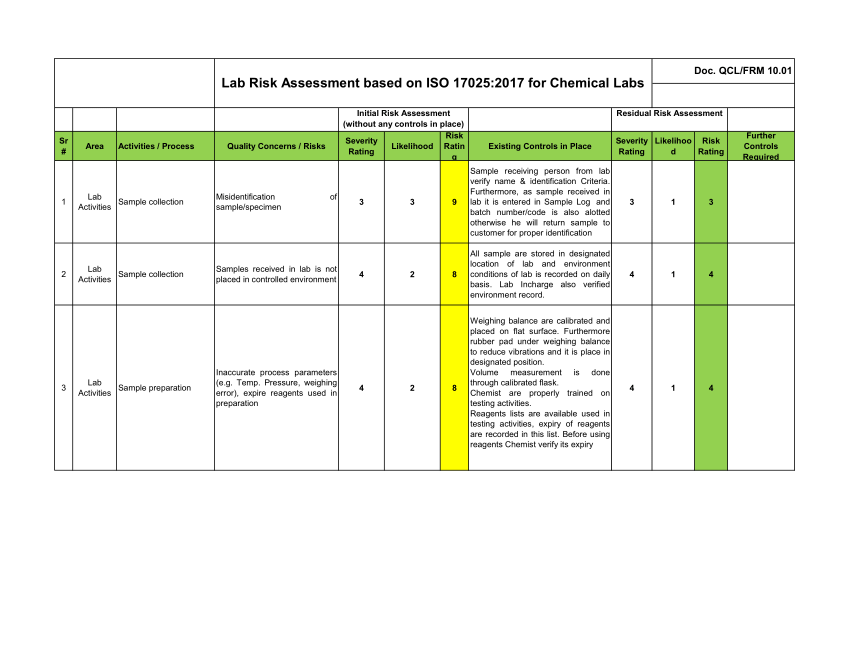
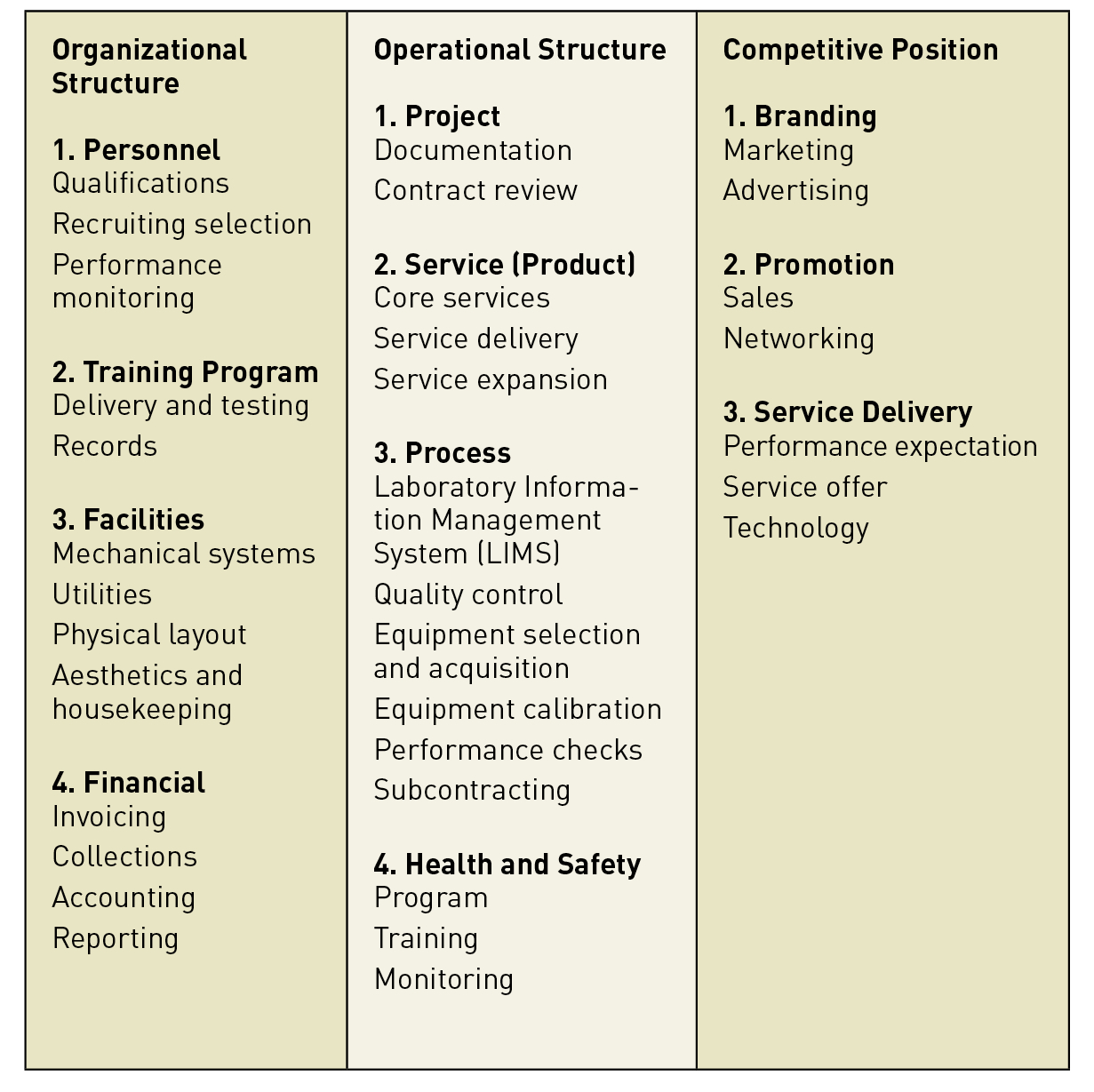
Application to Risk Management as Part of the Transition of the Quality Management System from ISO 17025 v2005 to ISO 17025 v2017: Case of MULTILAB Laboratory in Tunisia