Latent heat of vaporization as a function of (a) salinity (at 20 °C and... | Download High-Quality Scientific Diagram

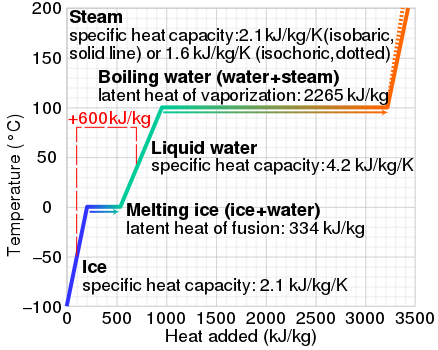
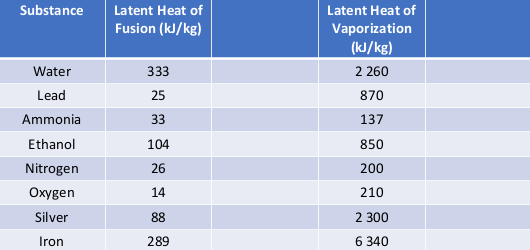
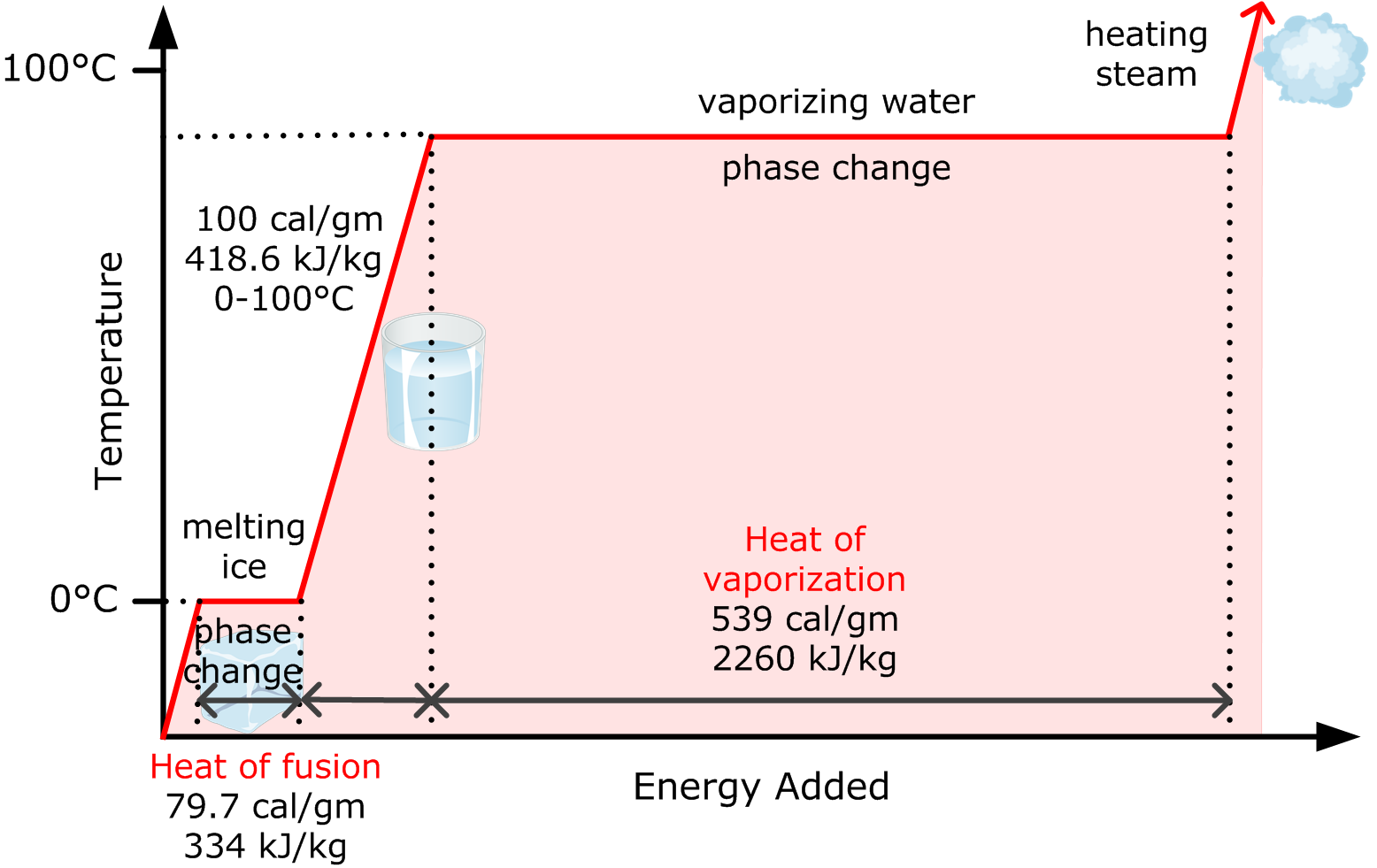
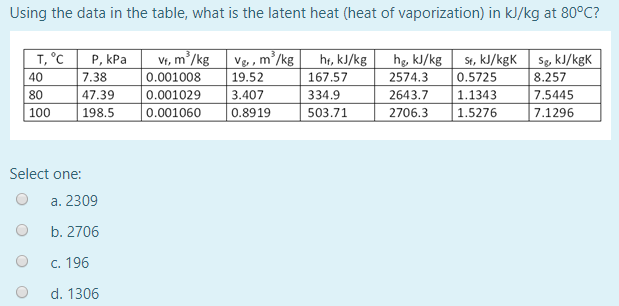
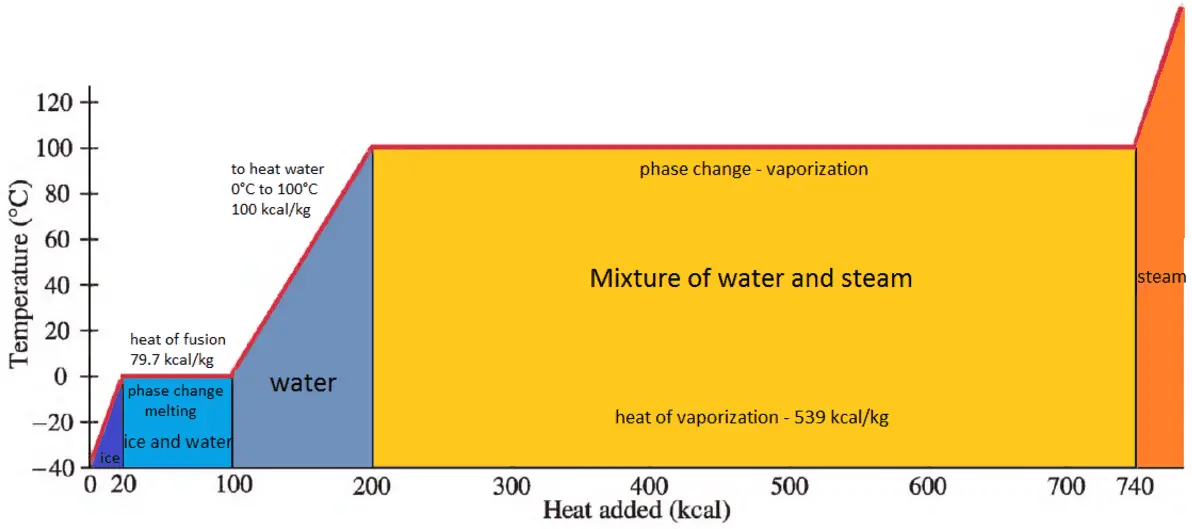
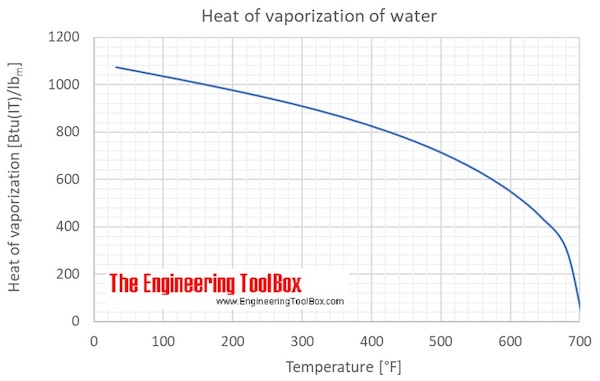
Water has the specific heat 4,186kJ/kg*""^(@)C, a boiling point of 100^(@)C, and a heat of vaporisation of 2,260kJ/kg. a sealed beaker contains 100g of water that's initially at 20^(@)C. If the water

Experimental and estimated values of the latent heat of vaporization of... | Download High-Quality Scientific Diagram





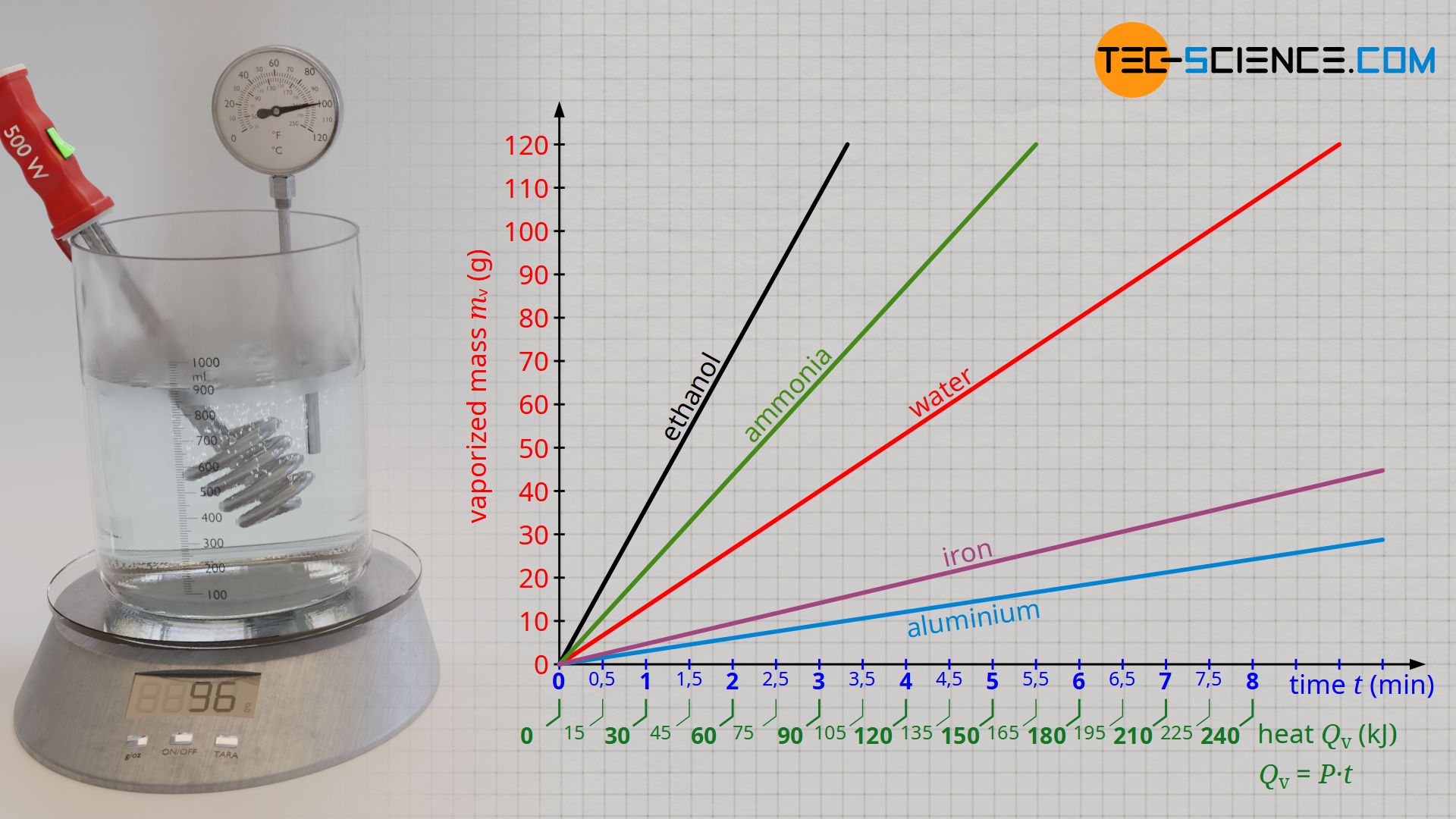
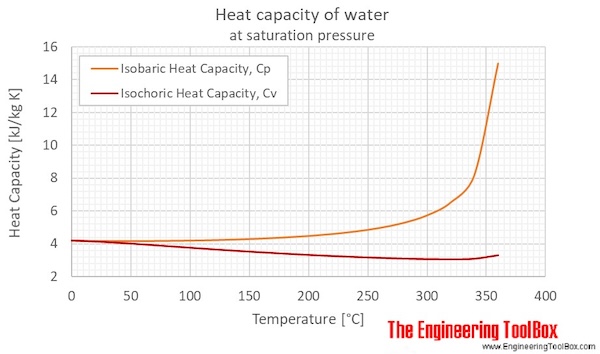
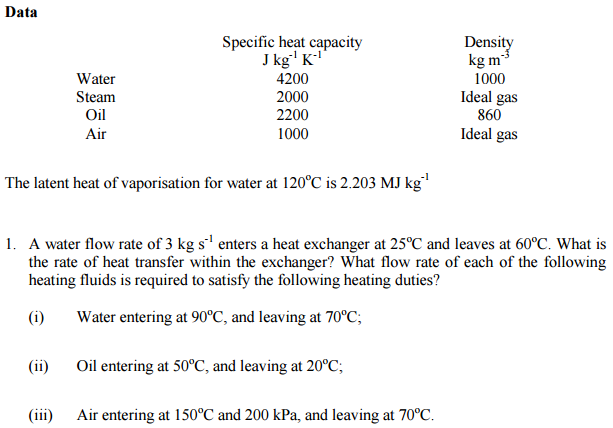
![Latent heat of vaporization for main components of LNG [10]. | Download Table Latent heat of vaporization for main components of LNG [10]. | Download Table](https://www.researchgate.net/publication/330572654/figure/tbl3/AS:718422421803010@1548296661881/Latent-heat-of-vaporization-for-main-components-of-LNG-10.png)